 Pharmacology & Pharmacy, 2012, 3, 474-480 http://dx.doi.org/10.4236/pp.2012.34065 Published Online October 2012 (http://www.SciRP.org/journal/pp) 1 Effects of Long-Term Use of Flavonoids on the Absorption and Tissue Distribution of Orally Administered Doses of Trace Elements in Rats Ausama Ayoob Jaccob1, Saad Abdulrahman Hussain2* 1Department of Pharmacology and Toxicology, College of Pharmacy, Al-Basra University, Basra, Iraq; 2Department of Pharma- cology and Toxicology, College of Pharmacy, University of Baghdad, Baghdad, Iraq. Email: *saad_alzaidi@yahoo.com Received July 26th, 2012; revised August 28th, 2012; accepted September 12th, 2012 ABSTRACT The risk of pharmacokinetic polyphenols-trace elements interaction may undesirable therapeutic outcomes. We evaluate the long-term use of silibinin, epigallo catechin (ECGC), quercetin and rutin on the absorption and tissue distribution of zinc, copper and iron after single oral doses in rats. Five groups of rats received either with olive oil as control or one of the polypheno ls silibinin, EPGC, quercetin o r rutin, administered orally as oily so lutions for 30 days. At day 30, a so lu- tion contains sulphate salt of zinc, copper and iron was administered orally; 3 hrs later blood samples, tissues of brain, kidney and liver were obtained for evaluation of the elements levels. The results showed that the polyphenols increased both serum and tissue levels of these elements compared with controls. This effect was relatively varied according to the structural differences among flavonoids. In conclusion, long-term use of supraphysiological doses of flavonoids increase absorptio n of Zn, Cu and Fe and their tissue availability in brain, kidney and liver; this effect seems to be dif- ferent with variations in structural features. Keywords: Flavonoids; Trace Elements; Absorption; Tissue Distribution 1. Introduction Flavonoids (polyphenolic compounds) are one of the bio- active compounds widely availa ble i n fruit s and veget a bl es [1]. Flavonoids have long been associated with a variety of biochemical and pharmacological properties, including antioxidant, antiviral, anticarcinogenic, and anti-inflam- matory activities [2], and believed to be beneficial to human health. Many peoples are motivated by scientific research that is widely carried in the news media, which indicated these flavonoids and polyphenols could prevent cancer, ageing, and cardiovascular diseases [3,4]. How- ever, these researches are often carried out in animals and their effects in humans remain uncertain [5]. A large body of evidence, mainly derived from preclinical studies in animals, has concluded that dietary polyphenols, when given in large qu antities, can ha ve desirab le o utco mes [6]. There is currently an extensive range of flavonoid sup- plements on the market [7]. Su ppliers of su ch s uppl emen ts recommend daily flavonoid intakes in amounts that are many times higher than those doses which can normally be achieved from a flavonoid-rich diet. The question arises whether supplements containing such supra-physio- logical flavonoid levels may exhibit adverse effects. In addition, it is likely th at a large proportion of individuals taking dietary flavonoid supplements are also taking con- ventional drugs or trace elements [8]. The concomitant intake of “supra-nutritional” flavonoid doses together with conventional drugs may lead to flavonoid-drug inter- actions [8]. Approximately 38 million adults in the US (18.9% of the population) use herbal p roducts that contain flavonoids or other natural supplements, but only one third tell their physician about this use [9]. This lack of information, combined with the fact th at natural products are usually a mixture of many active ingredients, in- creases the likelihood of harm. Moreover, this additionally raises concerns about the safe use of dietary flavonoids. The risk of pharmacokinetic polyphenols-trace elements interaction poses two major extremity challenges, phar- macotoxicity and treatment failure. The former can result from the inhibition of the homeostatic mechanisms responsible for the absorption, tissue distribution and clearance of the trace elements, while the latter may be the consequence of inducing processes the lead to faster clearance. This is in addition to the intrinsic pharma- codynamic actions of the polyphenols themselves which may include potentiating, additive, antagonism, or neu- *Corresponding a uthor. Copyright © 2012 SciRes. PP  Effects of Long-Term Use of Flavonoids on the Absorption and Tissue Distribution of Orally Administered Doses of Trace Elements in Rats 475 tralization effects. The present study was designed to evaluate the effect of long-term use of supraphysiological doses of silibinin, epigallocatechin gallate, quercetin and rutin on the absorption and tissue distribution of orally administered doses of the trace elements zinc copper and iron in rats. 2. Materials and Methods 2.1. Chemicals and Reagents Silibinin dihemisuccinate (SDH) (98% purity) was ob- tained from Tolbiac SRL, Argentina; Quercetin d ihydrate (98% pure standardized extract) was purchased from Xian Co, China; Epigallocatechin gallate (EGCG) was a gift from Al-Razi Pharm Ind, Syria; Rutin was obtained from Merck Laboratories, Germany; Ferrous sulphate, Copper sulphate and Zinc sulphate were obtained from SD Fine Chemicals, India. 2.2. Animals and Study Design Thirty male adult Sprague Dawly rats of body weight 200 - 250 g were obtained from the Animal House, Department of Pharmacology and Toxicology, College of Pharmacy, Baghdad University, and the experiments were carried out in Department of Pharmacology, College of Pharmacy, Al-Basra University, Iraq. The rats were housed under controlled conditions (22˚C - 25˚C) on a 1 2 h light/12 h dark cycle, and received the standard pellet diet (National Center for Drug Research and Quality Control, Baghdad) and water ad libitum. The study protocol was approved by the Institutional Animal Ethi cal Committee (IAEC), College of Pharmacy, University of Baghdad. After acclimatization for a period of one week, the animals were allocated into five groups consisting of 6 rats each; first group was treated with vehicle (olive oil) as control group; the other four groups are treated with one of the flavonoids: SDH (100 mg/kg), EGCG (25 mg/kg); Quercetin (50 mg/kg) and Rutin (500 mg/kg). All flavonoids are prepared as o ily solutions dissolved in olive oil and introduced as single daily doses admi- nistered orally using gavage tube for 30 consecutive days; the control group receives 0.2 mL/day of olive oil in the same way. At day 30, all groups of rats received orally single doses of Zinc sulphate (60 mg/kg), Copper sulphate (60 mg/kg) and Fe sulphate (60 mg/kg), all these ele men ts were administered 2 hrs after administration of the last doses of the flavonoids and the vehicle. 2.3. Samples Preparation After 3.0 hrs of administration of trace metals, all ani- mals are sacrificed after short duration anesthesia with anesthetic ether; blood samples were dra w n and collected in polyethylene tube, centrifuged at 10000 rpm for 20 min and the resulted serum was kept frozen at –20˚C until trace elements analysis. The liver and both kidneys were quickly removed and perfused with ice-cooled sa- line; the brain was carefully excised, rinsed with ice- cooled saline and the arachnoid membrane was carefully removed. One gram tissue of the obtained organs and 1.0 ml of serum were digested utilizing the wet digestion method [10,11]; the digested samples were stored in re- frigerator and used later for analysis of tissue and serum levels of zinc, copper and iron [12]. 2.4. Analysis of Trace Elements The contents of Zn, Cu and Fe in serum and tissue samples were first released from the protein matrix by wet digestion method as mentioned previously, and their concentrations were determined using atomic absorption spectrophotometer (Buck Scientific, Model 211-VGI, USA) at wavelength of 214 nm for zinc, 247 nm for Fe and 324 for Cu [13]. Standard solutions of these elements were used to prepare calibration carve for quantitative analysis. 2.5. Statistical Analysis Values were expressed as mean ± S.D; the values were statistically evaluated using unpaired Student's t-test and one way analysis of variance (ANOVA), supported by Bonferroni’s post hoc analysis. Values with P < 0.05 were considered significantly different. Analysis was performed using GraphPad Prism software for Windows (version 5.0, Grap hPad Software, Inc., San Die g o, CA) . 3. Results Figure 1 showed that all the flavonoids used in the pre- sent study significantly increased (P < 0.05) the GI ab- sorption of Zn, when administered as single oral dose compared to control group; meanwhile, no significant differences in serum Zn levels were reported among the effect of the four flavonoids (P > 0.05) in this respect. Concerning long-term effects of the tested flavonoids on the absorption of Cu, all of them produced significant increase (P < 0.05) in serum Cu levels compared to the values reported in controls; only SDH and quercetin demonstrated significantly different effects in this respect (lower effect for quercetin), when the effects of the four flavonoids compared with each others (Figure 2). In Figure 3, treatment of rats with one of the four flavon- oids used in the study for 30 days resulted in significant increase (P < 0.05) in the oral absorption of Fe when administered as single dose of ferrous sulphate compared to control group. When the effects of the studied flavon- Copyright © 2012 SciRes. PP 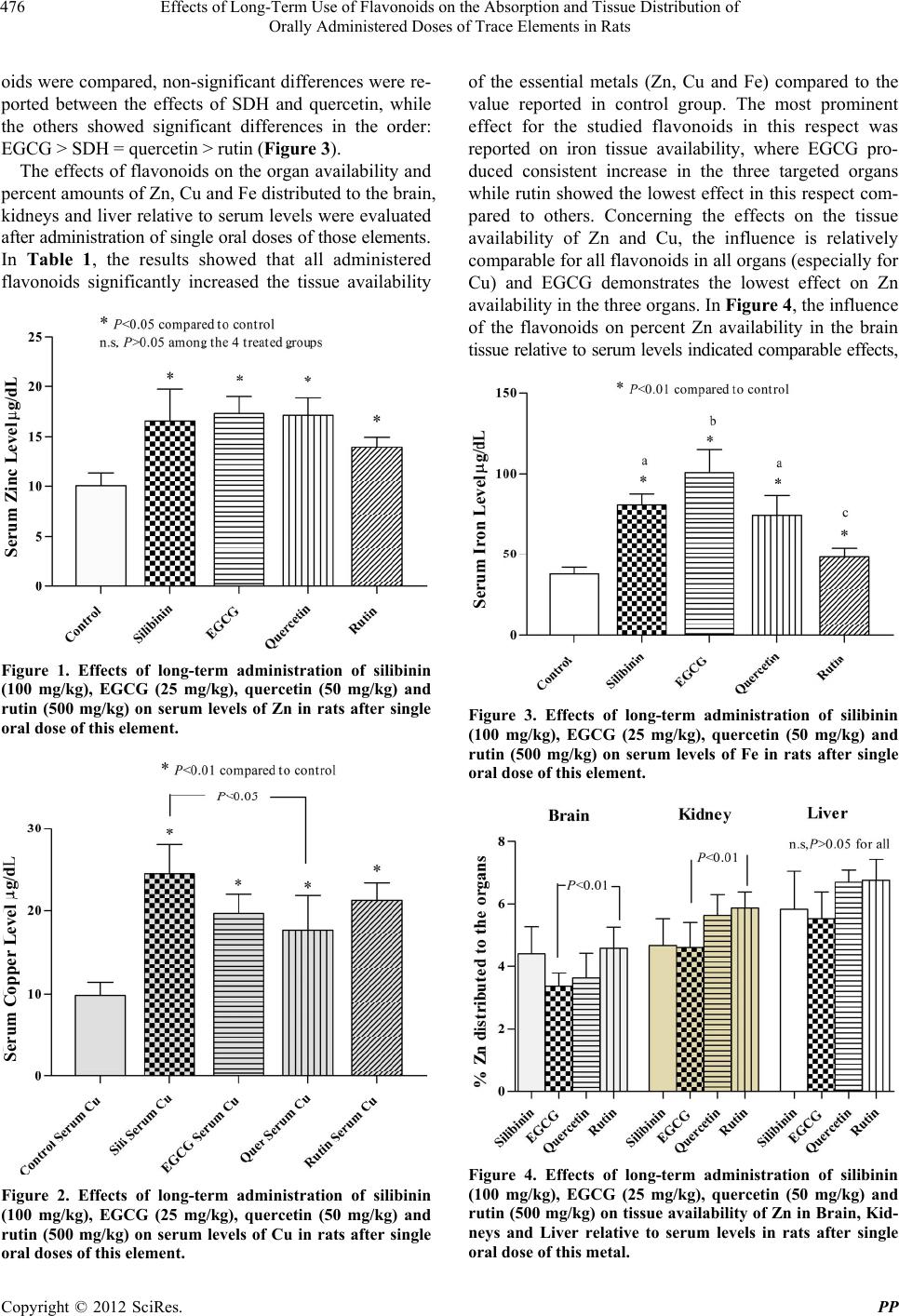 Effects of Long-Term Use of Flavonoids on the Absorption and Tissue Distribution of Orally Administered Doses of Trace Elements in Rats 476 oids were compared, non-significant differences were re- ported between the effects of SDH and quercetin, while the others showed significant differences in the order: EGCG > SDH = quercetin > rutin (Figure 3). The effects of flavonoids on the organ availability and percent amounts of Zn, Cu and Fe distributed to the brain, kidneys and liver relative to serum levels were evalu ated after administration of single oral doses of those elements. In Table 1, the results showed that all administered flavonoids significantly increased the tissue availability Serum Zinc Level Figure 1. Effects of long-term administration of silibinin (100 mg/kg), EGCG (25 mg/kg), quercetin (50 mg/kg) and rutin (500 mg/kg) on serum levels of Zn in rats after single oral dose of this element. Serum Copper Level Figure 2. Effects of long-term administration of silibinin (100 mg/kg), EGCG (25 mg/kg), quercetin (50 mg/kg) and rutin (500 mg/kg) on serum levels of Cu in rats after single oral doses of this element. of the essential metals (Zn, Cu and Fe) compared to the value reported in control group. The most prominent effect for the studied flavonoids in this respect was reported on iron tissue availability, where EGCG pro- duced consistent increase in the three targeted organs while rutin showed the lowest effect in this respect com- pared to others. Concerning the effects on the tissue availability of Zn and Cu, the influence is relatively comparable for all flavonoids in all organs (especially for Cu) and EGCG demonstrates the lowest effect on Zn availability in the three organs. In Figure 4, the influence of the flavonoids on percent Zn availability in the brain tissue relative to serum levels indi cated comparable effects, Serum Iron Level Figure 3. Effects of long-term administration of silibinin (100 mg/kg), EGCG (25 mg/kg), quercetin (50 mg/kg) and rutin (500 mg/kg) on serum levels of Fe in rats after single oral dose of this element. Figure 4. Effects of long-term administration of silibinin (100 mg/kg), EGCG (25 mg/kg), quercetin (50 mg/kg) and rutin (500 mg/kg) on tissue availability of Zn in Brain, Kid- neys and Liver relative to serum levels in rats after single oral dose of this metal. Copyright © 2012 SciRes. PP 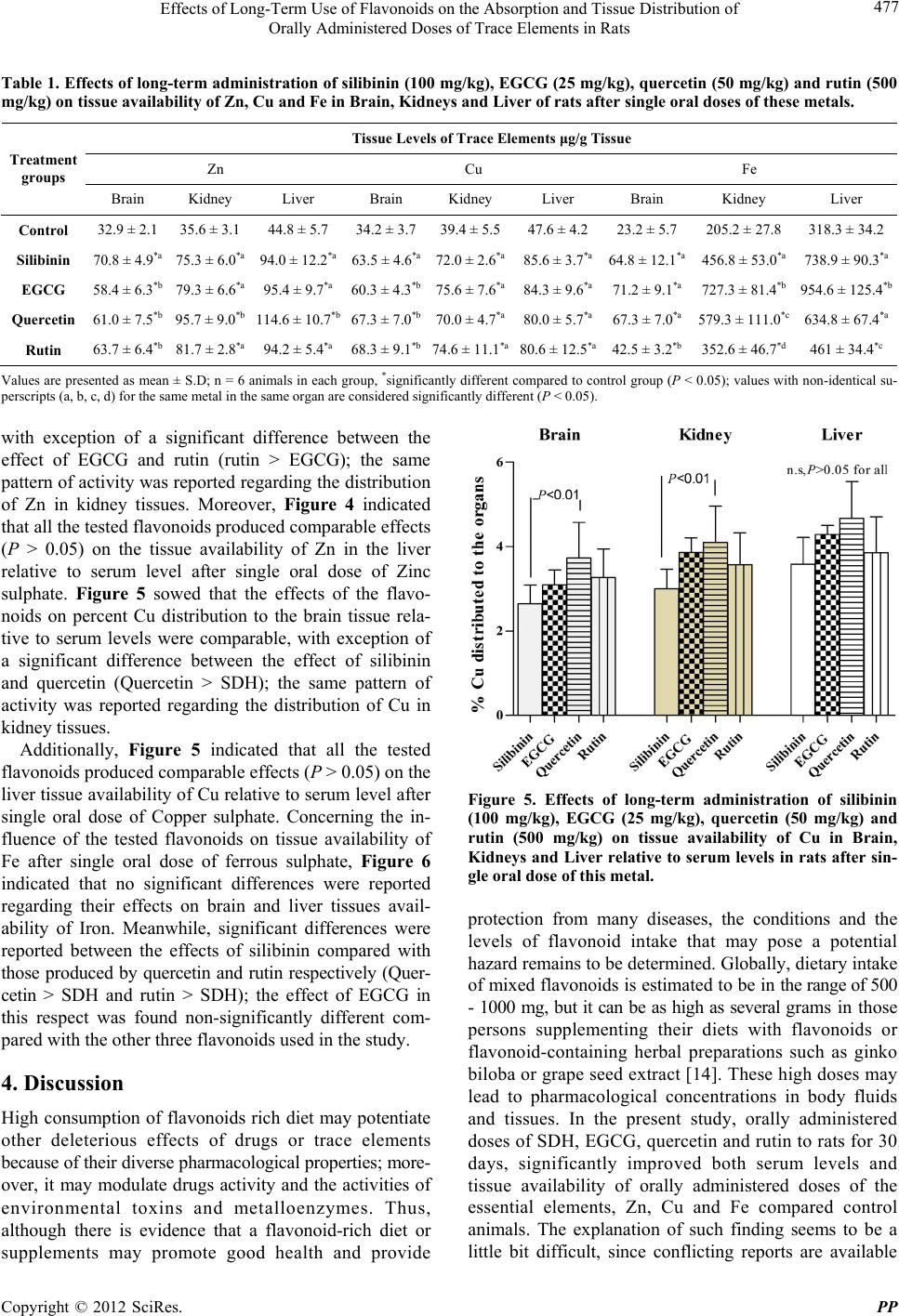 Effects of Long-Term Use of Flavonoids on the Absorption and Tissue Distribution of Orally Administered Doses of Trace Elements in Rats Copyright © 2012 SciRes. PP 477 Table 1. Effects of long-term administration of silibinin (100 mg/kg), EGCG (25 mg/kg), quercetin (50 mg/kg) and rutin (500 mg/kg) on tissue availability of Zn, Cu and Fe in Brain, Kidneys and Liver of rats after single oral doses of these metals. Tissue Levels of Trace Elements μg/g Tissue Zn Cu Fe Treatment groups Brain Kidney Liver Brain Kidney Liver Brain Kidney Liver Control 32.9 ± 2.1 35.6 ± 3.1 44.8 ± 5.7 34.2 ± 3.739.4 ± 5.547.6 ± 4.223.2 ± 5.7 20 5.2 ± 27.8 318.3 ± 34. 2 Silibinin 70.8 ± 4.9*a 75.3 ± 6.0*a 94.0 ± 12.2*a 63.5 ± 4.6*a 72.0 ± 2.6*a 85.6 ± 3.7*a 64.8 ± 12.1*a 456.8 ± 53.0*a 738.9 ± 90.3*a EGCG 58.4 ± 6.3*b 79.3 ± 6.6*a 95.4 ± 9.7*a 60.3 ± 4. 3*b75.6 ± 7.6*a 84.3 ± 9.6*a 71.2 ± 9.1*a 727.3 ± 81.4*b 954.6 ± 125.4*b Quercetin 61.0 ± 7 .5*b 95.7 ± 9.0*b 114.6 ± 10.7*b 67.3 ± 7.0*b 70.0 ± 4.7*a 80.0 ± 5.7*a 67.3 ± 7.0*a 579.3 ± 111.0*c 634.8 ± 67.4*a Rutin 63.7 ± 6.4*b 81.7 ± 2.8*a 94.2 ± 5.4*a 68.3 ± 9.1*b 74.6 ± 11.1*a80.6 ± 12.5*a42.5 ± 3 .2*b 352.6 ± 46.7*d 461 ± 34.4*c Values are presented as mean ± S .D; n = 6 animals in each gro up, *significantly different compared to control group (P < 0.05); values with non-identical su- perscripts (a, b, c, d) for the same metal in the same organ are considered significantly different (P < 0.05). with exception of a significant difference between the effect of EGCG and rutin (rutin > EGCG); the same pattern of activity was reported reg arding the distribu tion of Zn in kidney tissues. Moreover, Figure 4 indicated that all the tested flavono ids produced comparable effects (P > 0.05) on the tissue availability of Zn in the liver relative to serum level after single oral dose of Zinc sulphate. Figure 5 sowed that the effects of the flavo- noids on percent Cu distribution to the brain tissue rela- tive to serum levels were comparable, with exception of a significant difference between the effect of silibinin and quercetin (Quercetin > SDH); the same pattern of activity was reported regarding the distribution of Cu in kidney tissues. Additionally, Figure 5 indicated that all the tested flavonoids produced comparable effects (P > 0.05) on the liver tissue availability of Cu relative to serum level after single oral dose of Copper sulphate. Concerning the in- fluence of the tested flavonoids on tissue availability of Fe after single oral dose of ferrous sulphate, Figure 6 indicated that no significant differences were reported regarding their effects on brain and liver tissues avail- ability of Iron. Meanwhile, significant differences were reported between the effects of silibinin compared with those produced by quercetin and rutin respectively (Quer- cetin > SDH and rutin > SDH); the effect of EGCG in this respect was found non-significantly different com- pared with the othe r th ree fl avon oi d s used in the study . Figure 5. Effects of long-term administration of silibinin (100 mg/kg), EGCG (25 mg/kg), quercetin (50 mg/kg) and rutin (500 mg/kg) on tissue availability of Cu in Brain, Kidneys and Liver relative to serum levels in rats after sin- gle oral dose of this metal. protection from many diseases, the conditions and the levels of flavonoid intake that may pose a potential hazard remains to be determined. Globally, dietary intake o f m ix e d fl a vo n oi d s i s es t im a te d to b e i n the range of 500 - 1000 mg, but it can be as high as several gr ams i n t hose persons supplementing their diets with flavonoids or flavonoid-containing herbal preparations such as ginko biloba or grape seed extract [14]. These high doses may lead to pharmacological concentrations in body fluids and tissues. In the present study, orally administered doses of SDH, EGCG, quercetin and rutin to rats for 30 days, significantly improved both serum levels and tissue availability of orally administered doses of the essential elements, Zn, Cu and Fe compared control animals. The explanation of such finding seems to be a little bit difficult, since conflicting reports are available 4. Discussion High consumption of flavonoids rich diet may potentiate other deleterious effects of drugs or trace elements because of their diverse pharmacological properties; more- over, it may modulate drugs activity and the activities of environmental toxins and metalloenzymes. Thus, although there is evidence that a flavonoid-rich diet or supplements may promote good health and provide 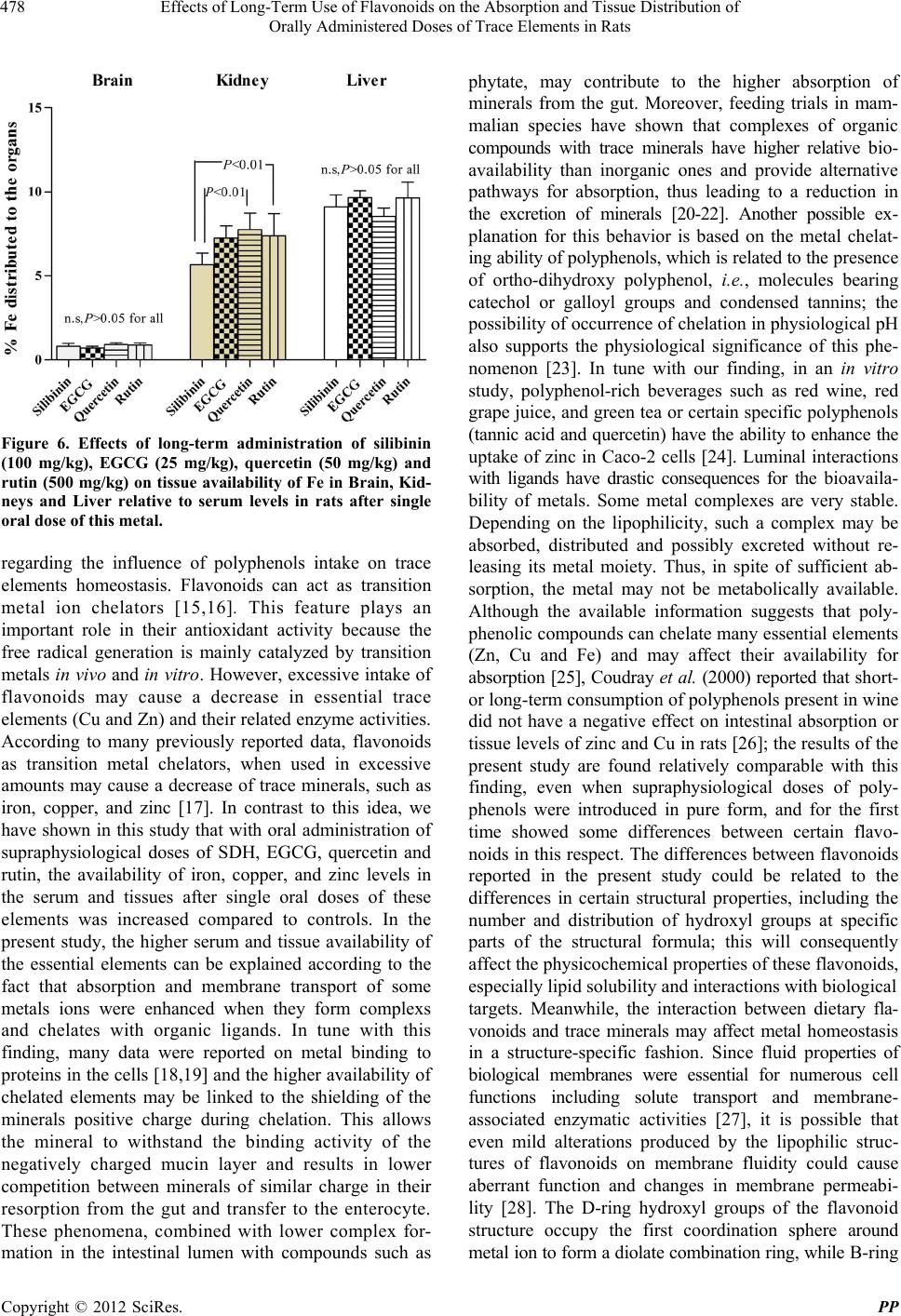 Effects of Long-Term Use of Flavonoids on the Absorption and Tissue Distribution of Orally Administered Doses of Trace Elements in Rats 478 Figure 6. Effects of long-term administration of silibinin (100 mg/kg), EGCG (25 mg/kg), quercetin (50 mg/kg) and rutin (500 mg/kg) on tissue availability of Fe in Brain, Kid- neys and Liver relative to serum levels in rats after single oral dose of this metal. regarding the influence of polyphenols intake on trace elements homeostasis. Flavonoids can act as transition metal ion chelators [15,16]. This feature plays an important role in their antioxidant activity because the free radical generation is mainly catalyzed by transition metals in vivo and in vitro. However, excessive intake of flavonoids may cause a decrease in essential trace elements (Cu and Zn) and their related enzyme activities. According to many previously reported data, flavonoids as transition metal chelators, when used in excessive amounts may cause a decrease of trace minerals, such as iron, copper, and zinc [17]. In contrast to this idea, we have shown in this study that with oral administration of supraphysiological doses of SDH, EGCG, quercetin and rutin, the availability of iron, copper, and zinc levels in the serum and tissues after single oral doses of these elements was increased compared to controls. In the present study, the higher serum and tissue availability of the essential elements can be explained according to the fact that absorption and membrane transport of some metals ions were enhanced when they form complexs and chelates with organic ligands. In tune with this finding, many data were reported on metal binding to proteins in the cells [18,19] an d the higher availability of chelated elements may be linked to the shielding of the minerals positive charge during chelation. This allows the mineral to withstand the binding activity of the negatively charged mucin layer and results in lower competition between minerals of similar charge in their resorption from the gut and transfer to the enterocyte. These phenomena, combined with lower complex for- mation in the intestinal lumen with compounds such as phytate, may contribute to the higher absorption of minerals from the gut. Moreover, feeding trials in mam- malian species have shown that complexes of organic compounds with trace minerals have higher relative bio- availability than inorganic ones and provide alternative pathways for absorption, thus leading to a reduction in the excretion of minerals [20-22]. Another possible ex- planation for this behavior is based on the metal chelat- ing ability of polyphenols, which is related to the pr es en c e of ortho-dihydroxy polyphenol, i.e., molecules bearing catechol or galloyl groups and condensed tannins; the possibility of occurren ce of chelation in physiolog ical pH also supports the physiological significance of this phe- nomenon [23]. In tune with our finding, in an in vitro study, polyphenol-rich beverages such as red wine, red grape juice, and green tea or certain specific polyphenols (tannic acid and quercetin) have the ability to enhance the uptake of zinc in Caco-2 cells [24]. Luminal interactions with ligands have drastic consequences for the bioavaila- bility of metals. Some metal complexes are very stable. Depending on the lipophilicity, such a complex may be absorbed, distributed and possibly excreted without re- leasing its metal moiety. Thus, in spite of sufficient ab- sorption, the metal may not be metabolically available. Although the available information suggests that poly- phenolic compounds can chelate many essential elements (Zn, Cu and Fe) and may affect their availability for absorption [25], Coudray et al. (2000) reported that shor t- or long-term consumption of polyphenols present in wine did not have a negative effect on intestinal absorption or tissue levels of zinc and Cu in rats [26]; the results of the present study are found relatively comparable with this finding, even when supraphysiological doses of poly- phenols were introduced in pure form, and for the first time showed some differences between certain flavo- noids in this respect. The differences between flavonoids reported in the present study could be related to the differences in certain structural properties, including the number and distribution of hydroxyl groups at specific parts of the structural formula; this will consequently affect the physicochemical properties of these flavonoids, especially lipid solubility and interactions with b iological targets. Meanwhile, the interaction between dietary fla- vonoids and trace minerals may affect metal homeostasis in a structure-specific fashion. Since fluid properties of biological membranes were essential for numerous cell functions including solute transport and membrane- associated enzymatic activities [27], it is possible that even mild alterations produced by the lipophilic struc- tures of flavonoids on membrane fluidity could cause aberrant function and changes in membrane permeabi- lity [28]. The D-ring hydroxyl groups of the flavonoid structure occupy the first coordination sphere around metal io n to form a diolate combina tion rin g, wh ile B - ri n g Copyright © 2012 SciRes. PP  Effects of Long-Term Use of Flavonoids on the Absorption and Tissue Distribution of Orally Administered Doses of Trace Elements in Rats 479 hydroxyl groups have weak interactions with metal ions [29]. As suggested by previous reports [30,31], the number of hydroxyl groups on the B-ring in many flavonoids structures, the presence of a galloyl moiety, and the steric characters on the D-ring could affect its affinity for the lipid bilayers. As a result of this, incor- poration of the flavonoids into the lipid bilayers was enhanced, which could lead to the formation of another ion channels after complexing of flavonoids with metal ions, which is expected to induce structural variation and influence the effects of structurally different flavonoids in this respect. In conclusion, long-term use of supra- physiological doses of flavonoids increase gastr oin test inal absorption of essential elements (Zn, Cu and Fe) and their tissue availability in brain, kidney and liver; this effect seems to be different with variations in structural features of the flavonoids. 5. Acknowledgements The presented data was abstracted from PhD thesis sub- mitted to the Department of Pharmacology and Toxico- logy, College of Pharmacy, University of Baghdad. The authors thank University of Baghdad for supporting the project. REFERENCES [1] S. Pascual-Teresa, D. A. Moreno and C. Garcia-Viguera, “Flavanols and Anthocyanins in Cardiovascular Health: A Review of Current Evidence,” International Journal Mo- lecular Sciences, Vol. 11, No. 4, 2010, pp. 1679-1703. doi:10.3390/ijms11041679 [2] E. Middleton, C. Kandaswami and T. C. Theoharides, “The Effects of Plant Flavonoids on Mammalian Cells: Implications for Inflammation, Heart Disease and Can- cer,” Pharmacological Reviews, Vol. 52, No. 4, 2000, pp. 673-751. [3] V. M. Adhami and H. Mukhtar, “Polyphenols from Green Tea and Pomegranate for Prevention of Prostate Cancer,” Free Radicals Research, Vol. 40, No. 10, 2006, pp. 1095- 1104. doi:10.1080/10715760600796498 [4] H. E. Seifried, D. E. Anderson, E. I. Fisher and J. A. Milner, “A Review of the Interaction among Dietary An- tioxidants and Reactive Oxygen Species,” Journal of Nu- trition and Biochemistry, Vol. 18, No. 9, 2007, pp. 567- 579. doi:10.1016/j.jnutbio.2006.10.007 [5] B. Halliwell, “Dietary Polyphenols: Good, Bad, or Indif- ferent for Your Health?” Cardiovascular Research, Vol. 73, No. 2, 2007, pp. 341-347. doi:10.1016/j.cardiores.2006.10.004. [6] S. C. Thomasset, D. P. Berry, G. Garcea, T. Marczylo, W. P. Steward and A. J. Gescher, “Dietary Polyphenolic Phytochemicals-Promising Cancer Chemopreventive Age n ts in Humans? A Review of Their Clinical Properties,” In- ternational Journal of Cancer, Vol. 120, No. 3, 2007, pp. 451-458. doi:10.1002/ijc.22419 [7] J. C. Espin, M. T. Garcia-Conesa and F. A. Tomas-Bar- beran, “Nutraceuticals: Facts and Fiction,” Phytochemis- try, Vol. 68, No. 22-24, 2007, pp. 2986-3008. doi:10.1016/j.phytochem.2007.09.014 [8] R. Cermak, “Effect of Dietary Flavonoids on Pathways Involved in Drug Metabolism,” Expert Opinion in Drug Metabolism and Toxicology, Vol. 4, No. 1, 2008, pp. 17-35. doi:10.1517/17425255.4.1.17doi:10.1093/ecam/nem045 [9] J. Kennedy, C. C. Wang and C. H. Wu, “Patient Disclo- sure about Herb and Supplement Use among Adults in the US,” Evidence Based Complement Alternative Medicine, Vol. 5, No. 4, 2008, pp. 451-456. [10] O. O. Babalola, R. E. Okonji, J. O. Atoyebi, T. F. Sen- nuga, et al., “Distribution of Lead in Selected Organs and Tissues of Albino Rats Exposed to Acute Lead Toxicity,” Scientific Research Essay, Vol. 5, No. 9, 2010, pp. 845- 848. [11] R. A. Jacob, H. H. Sandstead, J. M. Munoz, L. M. Klevay and D. B. Milne, “Whole Body Surface Loss of Trace Metals in Normal Males,” American Journal of Clinical Nutrition, Vol. 34, No. 7, 1981, pp. 1379-1383. [12] Z. Gao, H. Xu and K. Huang, “Effects of Rutin Supple- mentation on Antioxidant Status and Iron, Copper and Zinc Contents in Mouse Liver and Brain,” Biological Trace Elements Research, Vol. 88, No. 3, 2002, pp. 271- 279. doi:10.1385/BTER:88:3:271 [13] O. Akinloye, F. M. Abbiyesuku, O. O. Oguntibeju, A. O. Arowojolu and E. J. Truter, “The Impact of Blood and Seminal Plasma Zinc and Copper Concentrations on Spermogram and Hormonal Changes in Infertile Nigerian Men,” Reproductive Biology, Vol. 11, No. 2, 2011, pp. 83-98. [14] C. F. Skibola and M. T. Smith, “Potential Health Impacts of Excessive Flavonoid Intake,” Free Radicals in Biology and Medicine, Vol. 29, No. 3, 2000, pp. 375-383. doi:10.1016/S0891-5849(00)00304-X [15] I. Morel, G. Lescoat, P. Cognel, O. Sergent, N. Pasdelop, P. Brissot, et al., “Antioxidants and Iron-Iron-Chelating Activities of the Flavonoids Catechin, Quercetin and Di- osmetin on Iron-Loaded Rat Hepatocyte Cultures,” Bio- chemical Pharmacology, Vol. 45, No. 1, 1993, pp. 13-19. doi:10.1016/0006-2952(93)90371-3 [16] P. Sestili, A. Guidarelli, M. Dacha and O. Cantoni, “Quer- cetin Prevents DNA Single Strand Breakage and Cyto- toxicity Caused by Tert-Butylhydroperoxide: Free Radi- cal Scavenging Versus Iron Chelating Mechanism,” Free Radicals in Biology and Medicine, Vol. 25, No. 2, 1998, pp. 196-200. doi:10.1016/S0891-5849(98)00040-9 [17] S. Samman, B. Sandstrom, M. B. Toft, K. Bukhave, M. Jensen, S. S. Sorensen and M. Hansen, “Green Tea or Rosemary Extract Added to Foods Reduces Nonheme- Iron Absorption,” American Journal of Clinical Nutrition, Vol. 73, No. 3, 2001, pp. 607-612. [18] M. Murariu, E. S. Dragan and G. Drochioiu, “Electros- pray Ionization Mass Spectrometric Approach of Con- formationally-Induced Metal Binding to Oligopeptides,” European Journal of Mass Spectrometry, Vol. 16, No. 4, Copyright © 2012 SciRes. PP  Effects of Long-Term Use of Flavonoids on the Absorption and Tissue Distribution of Orally Administered Doses of Trace Elements in Rats Copyright © 2012 SciRes. PP 480 2010, pp. 511-521. doi:10.1255/ejms.1092 [19] G. Drochioiu, M. Manea, M. Dragusanu, M. Murariu, et al., “Interaction of Beta-amyloid(1-40) peptide with Pairs of Metal Ions: An Electrospray Ion Trap Ma ss S p ectro m et- ric Model Study,” Biophysical Chemistry, Vol. 144, No. 1-2, 2009, pp. 9-20. doi:10.1016/j.bpc.2009.05.008 [20] Y. M. Bao, M. Choct, P. A. Iji and K. Bruerton, “Effect of Organically Complexed Copper, Iron, Manganese and Zinc on Broiler Performance, Mineralexcretion and Ac- cumulation in Tissues,” Journal of Applied Poultry Re- search, Vol. 16, No. 3, 2007, pp. 448-455. [21] L. Nollet, J. D. van der Klis, M. Lensing and P. Spring, “The Effect of Replacing Inorganic with Organic Trace Minerals in Broiler Diets on Productive Performance and Mineral Excretion,” Journal of Applied Poultry Research, Vol. 16, No. 4, 2007, pp. 592-597. doi:10.3382/japr.2006-00115 [22] A. G. Abdallah, O. M. El-Husseiny and K. O. Abdel-Latif, “Influence of Some Dietary Organic Mineral Supple- mentations on Broiler Performance,” International Jour- nal of Poultry Sciences, Vol. 8, No. 3, 2009, pp. 291-298. doi:10.3923/ijps.2009.291.298 [23] M. Andjelkovic, J. van Camp, B. de Meulenaer, G. De- paemelaere, et al., “Iron-Chelation Properties of Phenolic Acids Bearing Catechol and Galloyl Groups,” Food Chemistry, Vol. 98, No. 1, 2006, pp. 23-31. doi:10.1016/j.foodchem.2005.05.044 [24] K. Sreenivasulu, P. Raghu and K. M. Nair, “Polyphenol- Rich Be verage s Enhance Zinc Upt ake an d Metal-Lo thionei n Expression in Caco-2 Cells,” Journal of Food Sciences, Vol. 75, No. 4, 2010, pp. H123-H128. doi:10.1111/j.1750-3841.2010.01582.x. [25] C. Coudray, C. Bousset, D. Pepin, J. C. Tressol, et al., “Short-Term Ingestion of Chlorogenic or Caffeic Acids Decreases Zinc But not Copper Absorption in Rats, Utili- zation of Stable Isotopes and Inductively Coupled Plasma Mass Spectrometry Technique,” British Journal of Nutri- tion, Vol. 80, No. 6, 1998, pp. 575-584. [26] C. Coudray, J. C. Tressol, C. Feillet-Coudray , J. Be l la ng er , et al., “Long-Term Consumption of Red Wine Does Not Modify Intestinal Absorption or Status of Zinc and Cop- per in Rats,” Journal of Nutrition, Vol. 130, No. 5, 2000, pp. 1309-1313. [27] D. G. Salinas, M. de La Fuente and J. G. Reyes, “Changes of Enzyme Activity in Lipid Signaling Pathways Related to Substrate Reordering,” Biophysics Journal, Vol. 89, No. 2, 2005, pp. 885-894. doi:10.1529/biophysj.104.057307 [28] N. T. de Gomez Dumm, A. M. Giammona, L. A. Touceda and C. Raimondi, “Lipid Abnormalities in Chronic Renal Failure Patients Undergoing Hemodialysis,” Medicina (B Aires), Vol. 61, No. 2, 2001, pp. 142-146. [29] R. E. Navarro, H. Santacruz and M. Inoue, “Complexation of Epigallocatechin Gallate (a Green Tea Extract, EGCG) with Mn2+: Nuclear Spin Relaxation by the Paramagnetic Ion,” Journal of Inorganic Biochemistry, Vol. 99, No. 2, 2005, pp. 584-588. doi:10.1016/j.jinorgbio.2004.11.013 [30] T. Hashimoto, S. Kumazawa, F. Nanjo, Y. Hara and T. Nakayama, “Interaction of Tea Catechins with Lipid Bi- layers Investigated with Liposome Systems,” Bioscience Biotechnology and Biochemistry, Vol. 63, No. 12, 1999, pp. 2252-2255. doi:10.1271/bbb.63.2252 [31] K. Kajiya, S. Kumazawa and T. Nakayama, “Steric Ef- Fects on Interaction of Tea Catechins with Lipid Bilay e r s , ” Bioscience Biotechnology and Biochemistry, Vol. 65, No. 12, 2001, pp. 2638-2643. doi:10.1271/bbb.65.2638.
|