 Vol.2, No.6, 600-6 11 (2010) Natural Science http://dx.doi.org/10.4236/ns.2010.26075 Copyright © 2010 SciRes. OPEN ACCESS Evolution of technogenic landscapes by the example of apatite-nepheline ore concentration wastes Vladimir N. Pereverzev1, Galina A. Evdokimova2*, Irina V. Zenkova2, Maria V. Korneykova2, Vera V. Redkina2 1Laboratory of Soil Science, Polar-Alpine Botanical Garden Institute, Kola Science Centre of Russian Academy of Sciences, Apatity, Russia 2Laboratory of Microorganisms Ecology, Institute of the North Industrial Ecology Problems, Kola Science Centre of Russian Acad- emy of Sciences, Apatity, Russia; *Corresponding Author: galina@inep.ksc.ru Received 10 February 2010; revised 30 April 2010; accepted 13 May 2010. ABSTRACT A primary soil-forming process can take place on the concentration waste of apatite-nepheline ores, whose biological recultivation was carried out more than 40 years ago. This process is characterized by the following features: forming of a thin litter with the content of organic carbon at the level of 8-12%, accumulation of humic substances in the sub-litter layer and the cha- nge of рН values. Microorganisms are biocata- lysts of primary soil formation processes and one of the main factors that determine the spe- cificity of this process. The prokaryotic complex of the newly formed soils, generated from neph- eline sands, is considerably different from that of zonal soils on moraine sediments. The former ones are dominated by gram-positive bacteria, mainly actinobacteria, as well as by their filam- entous forms (actinomycetes), whereas the lat- ter ones are dominated by gram-negative bac- teria. A common feature of invertebrate’s com- plexes in nepheline sands is the low species diversity, small-size and quickly development of microfauna and mesofauna representatives and the dependence of succession of microarthro- pods pioneer groups on the succession of bac- teria and fungi. Keywords: Soil-Forming Process ; Nepheline Sands; Organic Matter; Soil Biota 1. INTRODUCTION The mining and processing industry, which in the Mur- mansk region comprises several large enterprises, dam- ages natural landscapes both as a result of open mining operations and due to the generation of overburden and mineral concentration waste dumps. As a result of activ- ity of large concentration mills a large number of ore processing wastes are generated and stored in tailing dumps. The total area of territories covered by the tailing dumps in the Murmansk region at present makes about 5 thousand hectares. The operating and dormant tailing dumps represent a source of significant dusting of adja- cent areas, since erosion processes are much developed on them as well as in natural and man-induced deserts. On the other hand tailing dumps can be considered as man-induced deposits that can be used in the future for recovery of valuable elements as soon as new techniques are introduced. The investigation of primary soil formation processes, which take place on nepheline sands, was carried out from May to October 2005-2008 every years on tailing dumps with different duration of waste storage: 0, 10, 20, 30, 40 years. As a whole 180 sand samples have been taken for chemical and microbiological analyses and 300 samples for the zoological one. 2. NEPHELINE SANDS AS OBJECT OF BIOLOGICAL RECULTIVATION Nepheline sands, as wastes of apatite-nepheline ores concentration, represent a soil-forming rock which is untypical for cold damp conditions. Nepheline sands are similar to the widespread in the region moraine, sea and fluvioglacial sands, on which dominating Al-Fe-humus podzols were formed, only by their granulometric com- position. As well as in other soil-forming rocks, the mineral bulk of nepheline wastes comprise of fine sand fractions (0.25-0.05 mm – 29-31% in a layer of 0-15 sm) and coarse dust (0.05-0.01mm – 56-59%) with insigni- ficant content of slime particles (< 0.001 mm –  V. N. Pereverzev et al. / Natural Science 2 (2010) 600-611 Copyright © 2010 SciRes. OPEN ACCESS 2.4-3.0%). A peculiarity of granulometric composition of neph- eline sands is its heterogeneity with depths which is a consequence of influence of different conditions of de- posit accumulation in the course of tailing dump filling. By their bulk chemical composition nepheline sands significant differ from natural quaternary deposits (Ta- ble 1). The content of SiO2 in them can be an indicator of high-, or low-grade soil-forming rocks. It is natural that the less the content of this element in rock, the higher the content of other elements including the bio- genic ones, which determine the direction and intensity of biogeochemical processes in ecosystems. Sandy rocks with content of SiO2 of 80-85% belong to high grade rocks. In the Murmansk region the most widespread sea and continental rocks represented mainly by sands and sandy loams, differ from similar rocks of other northern re- gions by higher grade of chemical composition with the SiO2 content of 65-67%. Eluvium of nepheline syenites on which the soils of tundra belt of the Khibiny were generated is characterized by even higher grade compo- sition (SiO2 < 60%). The composition of nepheline sands usually includes about 40% of SiO2. They belong to very high grade rocks in their chemical composition. It con- tains considerable stocks of biogenic elements— phos- phorus and potassium. Since apatite-nepheline ore does not contain any quartz, Si in them is represented by sili- cates, by basically nepheline (NaAlSiO4). In the course of ore concentration the share of nepheline increases from 32 to 57%. The content of Р in nepheline sand is an order of mag- nitude higher than in moraine rocks since they contain apatite, not completely recovered in the process of ore concentration. Nepheline sands in terms of their supply with phosphorus also differ from cultivated soils both quantitatively, and qualitatively. They contain significa- ntly more total phosphorus, but phosphorus is repre- sented in them only by one composition—tricalcium phosphate (apatite). Table 1. Total chemical composition of nepheline and moraine sand, percentage on ignited sample. Rock SiO2 Al2O3 Fe2O3 CaO MgO sands 41.0 ± 0.6 21.0 ± 0.4 8.5 ± 0.3 6.5 ± 0.6 1.3 ± 0.1 sands 65.7 ± 1.3 13.8 ± 0.5 5.4 ± 0.3 4.1 ± 0.3 2.1 ± 0.2 Rock Ti O2 P2O5 MnO K2O Na2O Nepheline sands 2.6 ± 0.1 3.6 ± 0.6 0.18 ± 0.01 4.9 ± 0.1 10.7 ± 0.4 Moraine sands 0.9 ± 0.1 0.4 ± 0.1 0.12 ± 0.01 2.2 ± 0.3 3.7 ± 0.1 In soil, however, besides apatite, numerous other min- eral compositions of phosphorus are present and, besides, some part of phosphates is a part of organic composi- tions [1,2]. Apatite is weakly soluble at the impact of soil solutions, therefore, despite the considerable content of phosphorus in nepheline sands, the cultivation of pe- rennial grasses on them is impossible without introduc- ing phosphoric mineral fertilizers. The amount of potassium in tailings also exceeds that in moraine. On the average it makes 5%, which exceeds 2 times the content of this element in the cultivated podzolic soils [3]. Nepheline sands as a оbject of biological recultivation differ from zonal soils in the condition cation exchange capacity and the acid-base characteristics. If all soils generated on quaternary sediments are characterized by an acid medium, the nepheline sands have рН values in the alkaline range both in water, and salt suspensions (Table 2). In the course of long interaction of sands with the vegetative cover in the top part of mineral profile (to the depth of 20 sm) the reaction of medium in salt sus- pension changes into the acid range. While in deeper layers it remained alkaline. In water suspension the reac- tion of medium was alkaline at all depths. Other forms of acidity—the hydrolytic and the exchange ones—are also characterized by low indices not typical for zonal soils. Thus, the reaction of nepheline sands fundamentally differs from that of zonal soils. At the same time the distribution of рН values both in sands and in soils, which follows the general law—with depth the reaction of medium shifts towards neutral or alkaline values. It is in that the influence of eluvial processes tells on the mineral profile. Initial nepheline sands are devoid of organic sub- stance of biological origin. Presence of organic carbon in them is due to the remains of flotation reagents—a mix- ture of resin and fatty acids used in the technological process during that period. They are rather stable in time, which is confirmed by the presence of organic carbon in nepheline sands of 20-30-years “age”. The content of organic carbon of technogenic origin in sands makes 0.2-0.3%. Table 2. Average рН values in nepheline sands and podzols. Depth, sm рН (Н2О) рН (KСl) Soil on sands Podzol Soil on sands Podzol 0-1 7.1 4.3 5.8 3.5 1-5 7.5 4.7 6.2 3.8 5-10 7.6 5.1 6.4 4.6 20-60 8.2 5.5 7.1 5.0  V. N. Pereverzev et al. / Natural Science 2 (2010) 600-611 Copyright © 2010 SciRes. OPEN ACCESS Thus, nepheline, being the basis of mineral bulk of sands, is an unstable mineral which is exposed to inten- sive weathering under the impact of acid solutions, in- cluding humus acids. The extremely high mobility of biogenous elements which are a part of nepheline sands is connected with this [4]. The soil-forming rocks on which zonal soils of the Murmansk region were formed do not have similar properties in such pronounced form. On the other hand, the absence of organic substance and fixed nitrogen in nepheline sands, as well as the inacces- sibility of large reserves of phosphorus to plants requires solution of problems of optimization of the nutritive sta- tus of sands when growing on them plants for the nature protection and eventual economic purposes. Nepheline sands are suitable for cultivation of plants not only with a view of their fixation from wind erosion, but also for creation of productive agricultural lands, which proved true when carrying out field pilot works after growing meadow grasses on a fixed tailing dump [5]. 3. NEWLY FORMED SOILS ON NEPHELINE SANDS The studies of the structure and properties of soils for- med on vegetation fixed nepheline sands ware carried out on dormant tailing dump. Recultivation took place from 1964 to 1984 by sowing perenial grasses. Over a part of the tailing dump, recultivated in 1964-1968, a vegetative cover of various structures was generated de- pending on edaphic conditions, first of all, apparently, on the character of substrate humidification. Along with cereal grasses, some shrubs and subshrubs, motley gras- ses, in particular, red clover, participate in the formation of the ground cover, as well as mosses and lichens, typi- cal for zonal phytocenoses. The wood canopy is repre- sented by rare specimens of pine and spruce, planted in 1978 in the course of biological recultivation [6], and heavy birch thicket of 20-30-years age, which penetrated in the phytocenosis by natural seeding after the tailing dump surface fixation using perennial grasses. Some rare specimens of alder and juniper are found. Glades, devoid of tree and shrubs and herbaceous layer have a moss- lichen cover continuum. The soils inherited their total chemical composition from soil-forming rocks. The newly cultivated soils wh- ich age is estimated at several decades, can not essen- tially differ by their chemical composition from rocks on which they are formed. The rich chemical composition peculiar to rock is also characteristic for newly formed soils. The average for all sections content of SiO2 in soils on nepheline sands is 41% on ignited soil while in the arable layer of cultivated podzols it exceeds to 65%. The total content of Fe2O3 and Al2O3 reaches almost 30%, alkaline-earth bases—7.8%, alkaline metals— 15.6%, of them the share of Na2О is 10.7%. As said above, nepheline sands contain a lot of phosphorus as a part of apatite, which remained in tailings after the ore concentration. The reserves of phosphorus in soil, natu- rally, have been preserved. In nepheline sands and in soils generated on them, a high enough content (0.34% on average) of fluorine had been registered. In zonal podzols the content of this element usually made no more than 0.01-0.2% [7]. Fluorine is a part of apatite composition (a variety—fluorine-apatite) which is the reason of enrichment of nepheline sands with it. Thus the initial soil formation on nepheline sands proceeds in conditions of very rich chemical composition of soil- forming rocks. 3.1. Organic Matter of Newly Formed Soils Formation humus substances, specific organic com- pounds that are peculiar to soils, are the initial stage of soil profile formation. As a result of transformation by microorganisms of the vegetative litter on the surface of nepheline sands an organic horizon was generated. In connection with low power (0.5-1.5 sm) it was enriched by mineral particles. Therefore the content of organic carbon in it is relatively small—8-11 %. The average data of the content of organic carbon based on all investigated ecotopes are presented in Table 3. The greatest spatial variability of organic carbon value in organic horizon is noted (the variation coefficient makes 46%). In the top layers of mineral mass (in cespi- tose horizon and at the depth of 5-10 sm) the variability is insignificant (< 10%) and mean (10-20%) [8]. By this reason the difference in the content of organic carbon in these horizons is reliable. The difference of mean values was 0.2%, the error of difference was 0.031% and the Student criterion was 6.5 (t05 = 2.31). Hence, we can assert with sufficient reliability that under the influence of biota, which transforms plant residues, in the formed cespitose horizon an accumulation of organic substance took place in amounts exceeding its content in the initial nepheline sands. Table 3. The average content of organic carbon. Horizon (Depth, sm) Organic C, % Coefficient of variation, % АO (mulch) 7.66 ± 1.445 46 АY (cespitose) 0.54 ± 0.025 9 5-10 0.34 ± 0.019 13 10-20 0.31 ± 0.029 23 20-30 0.28 ± 0.025 22 30-40 0.28 ± 0.027 23  V. N. Pereverzev et al. / Natural Science 2 (2010) 600-611 Copyright © 2010 SciRes. OPEN ACCESS The content of water-soluble compounds of carbon, which is a part of organic substance of soils as a whole, is closely connected with the content of total carbon. To a greater extent, it concerns the organic horizon of soils, where rather a close correlation dependence of amount of water-soluble and total carbon (r = 0.728 ± 0.278 t = 2.81; t05 = 2.18) is found. In mineral layers of soils this connection is lesser significant (r = 0.384 ± 0.161; t = 2.38; t05 = 2.00). Probably, it is connected with the fact that in the mineral mass of sands the organic substance is represented both by compounds of biological nature and by nonspecific compounds—the remains of flotoreag- ents. In profile distribution of indices of water-soluble car- bon the same regularities, as in distribution of the total carbon are manifested (Table 4). It is natural, that the most of all water-soluble organic substance is found in organic horizon. In mineral horizons its amount de- creases rapidly, but in the cespitose layer (up to 5 sm) the content of water-soluble carbon is higher, than in the underlaying layers. Figure 1 presents the average data on all ecotopes, il- lustrating the regularities of profile distribution of the total and water-soluble carbon. Their absolute content follows the general regularity: rapidly decrease in direction from organic horizon to the cespitose one and the further gradual decrease with de- pth. Concerning the values of the degree of mobility of organic substance (of the content of water-soluble car- bon in percents of the total) the pattern is reverse. In direction from organic horizon to the cespitose one this value increases and continues to increase in the deeper layers. Hence, in the process of decrease in the content of organic substance with depth its relative mobility in- creases. The registered regularities are well described by exponential curves with rather a high approximation reliability (R2 = 0.88-0.90). By the fractional structure of humus acids soils on nepheline sands differ from zonal soils: in the composi- tion of humic and fulvic acids the fractions connected with calcium is played an appreciable role. It is caused by abundance of bases in nepheline sands, including the ones in mobile condition. 3.2. Acid-base Properties of Newly Formed Soils Acidity is an important indicator of soils fertility, formed as a result of interaction of plants with soil-forming rock, which in our case is represented by nepheline sands. Initial sands have alkaline reaction both in water (рН 8.0-8.3) and in salt (рН 7.3-7.8) suspensions. In the prosess of primary soil formation on the surface of nepheline sands covered with plants, a thin organic horizon there was generated, in which processes of transformation of plant litter take place, resulting in the formation of organic acids which interact with mineral mass of sands. The result of such interaction is accumu- lation of newly formed soil of organic mineral com- pounds being of acid nature in the top part of a mineral profile. The results of рН determination in water suspen- sion show, that an organic horizon has, as a rule, neutral reaction, and in a salt suspension the reaction of this horizon becomes subacidic (Table 5). Table 4. The average content of water-soluble carbon. Horizon (Depth, sm) Water-soluble C, mg/100 g Coefficient of variation, % AO 99 ± 10.8 26.9 АY (cespitose) 18 ± 0.9 9.4 5-10 11 ± 2.2 49.8 10-20 9 ± 2.1 57.9 20-30 11 ± 1.9 43.5 30-40 11 ± 2.7 59.4 Table 5. Average рН values in nepheline sand and podzols. Depth, sm рН (Н2О) рН (КСl) Soil on sands Podzol Soil on sands Podzol 0-1 7.1 4.3 5.8 3.5 1-5 7.5 4.7 6.2 3.8 5-10 7.6 5.1 6.4 4.6 20-60 8.2 5.5 7.1 5.0 Figure 1. Distribution of total carbon over soil profile (A); % of water-soluble carbon, mg/100 g (B); wa- ter-soluble carbon, % of total carbon (C). Average data. 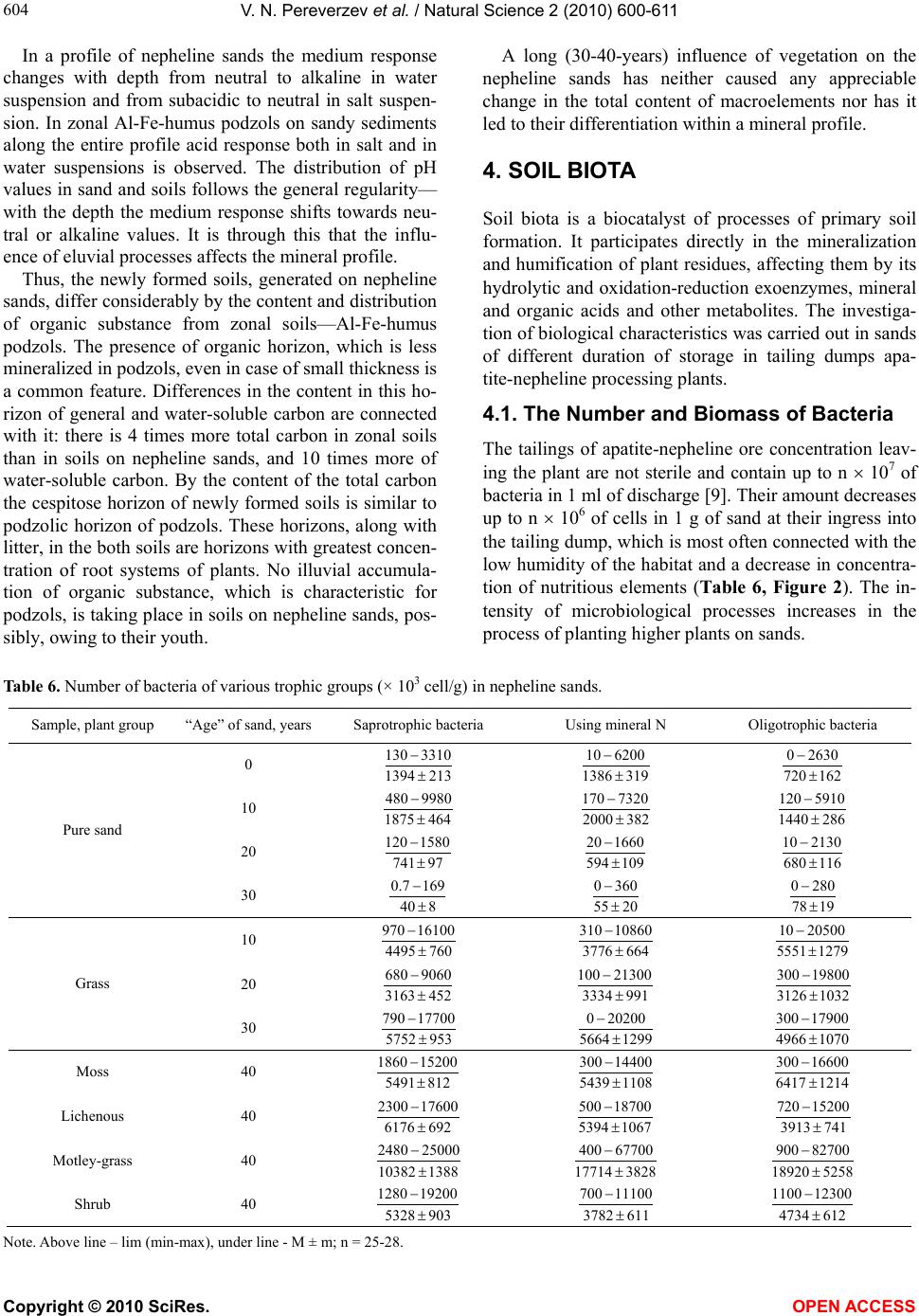 V. N. Pereverzev et al. / Natural Science 2 (2010) 600-611 Copyright © 2010 SciRes. OPEN ACCESS In a profile of nepheline sands the medium response changes with depth from neutral to alkaline in water suspension and from subacidic to neutral in salt suspen- sion. In zonal Al-Fe-humus podzols on sandy sediments along the entire profile acid response both in salt and in water suspensions is observed. The distribution of рН values in sand and soils follows the general regularity— with the depth the medium response shifts towards neu- tral or alkaline values. It is through this that the influ- ence of eluvial processes affects the mineral profile. Thus, the newly formed soils, generated on nepheline sands, differ considerably by the content and distribution of organic substance from zonal soils—Al-Fe-humus podzols. The presence of organic horizon, which is less mineralized in podzols, even in case of small thickness is a common feature. Differences in the content in this ho- rizon of general and water-soluble carbon are connected with it: there is 4 times more total carbon in zonal soils than in soils on nepheline sands, and 10 times more of water-soluble carbon. By the content of the total carbon the cespitose horizon of newly formed soils is similar to podzolic horizon of podzols. These horizons, along with litter, in the both soils are horizons with greatest concen- tration of root systems of plants. No illuvial accumula- tion of organic substance, which is characteristic for podzols, is taking place in soils on nepheline sands, pos- sibly, owing to their youth. A long (30-40-years) influence of vegetation on the nepheline sands has neither caused any appreciable change in the total content of macroelements nor has it led to their differentiation within a mineral profile. 4. SOIL BIOTA Soil biota is a biocatalyst of processes of primary soil formation. It participates directly in the mineralization and humification of plant residues, affecting them by its hydrolytic and oxidation-reduction exoenzymes, mineral and organic acids and other metabolites. The investiga- tion of biological characteristics was carried out in sands of different duration of storage in tailing dumps apa- tite-nepheline processing plants. 4.1. The Number and Biomass of Bacteria The tailings of apatite-nepheline ore concentration leav- ing the plant are not sterile and contain up to n × 107 of bacteria in 1 ml of discharge [9]. Their amount decreases up to n × 106 of cells in 1 g of sand at their ingress into the tailing dump, which is most often connected with the low humidity of the habitat and a decrease in concentra- tion of nutritious elements (Table 6, Figure 2). The in- tensity of microbiological processes increases in the process of planting higher plants on sands. Table 6. Number of bacteria of various trophic groups (× 103 cell/g) in nepheline sands. Sample, plant group “Age” of sand, years Saprotrophic bacteri a Using mineral N Oligotrophic bacteria Pure sand 0 10 20 30 Grass 10 20 30 Moss 40 Lichenous 40 Motley-grass 40 Shrub 40 Note. Above line – lim (min-max), under line - M ± m; n = 25-28.  V. N. Pereverzev et al. / Natural Science 2 (2010) 600-611 Copyright © 2010 SciRes. OPEN ACCESS Figure 2. The number of bacteria in non-recultivated nepheline sands of different storage duration and in sand under cereals. The highest number of all the trophic bacterial groups was reached under grass parcels. So, in a microbocenos developed in the thin organic horizon, generated at a nepheline tailing dump revege- tated 40 years ago, the leading position in which was occupied by oligotrophic bacteria and bacteria trans- forming complex organic nonnitrogenous substances, in particular, representatives of amylolytic community. The number of all trophic groups of bacteria was the greatest under of grass parcel. More complete data about the number of microorgan- isms in soils can be obtained using microscopic counts methods, in particular, the method of fluorescent micro- scopy. Based on the data of the total content of bacteria in the substrate, it is possible to calculate their biomass. The total number of bacteria in pure sand using the method of fluorescent microscopy, which considers both viable and unviable cells, varied within 0.34-0.60 billion cell/g, and in the recultivated one under various plant gropes—from 5.8 to 7.2 billion cell/g (Table 7). The “age” of pure, unrecultivated sand practically did not exert any influence on the total number of bacteria and their biomass. In sands under grass plants the bacterial biomass increased on the average 4 times compared to the sand which was not covered with plants. At that, no reliable changes in the amount of bacteria biomass occur as the age of the sand increases. The greatest number and biomass of bacteria was under motley grasses, do- minating in which was clover, capable to symbiotic fixa- tion of nitrogen. Thus, in the recultivated sands the bacterial biomass has increased on the average 14 times in comparison with the sands not covered with vegetation, and changed under various plants groups within 0.11-0.29 mg/g. 4.2. Peculiarities of the Prokaryotic Complex of Newly Formed Soils The prokaryotic complex of newly formed soils on nep- heline sands essentially differs from the prokaryotic complex of zonal soils on moraine. In the prokaryotic complex of the studied substrate gram-positive bacteria dominate, whereas in zonal soils gram-negative bacteria prevail which testifies to differences in the species composition of the bacterial community. In non-recul- tivated sands the share of gram-negative bacteria changed from 4 to 10% of the total number of organo- trophic bacteria. In the recultivated sands their share Table 7. The total number of bacteria (× 109 cells/g) and their biomass (× 10-5 g/g) in nepheline sands. Sample, plant group “Age” of sand, years Number Biomass Pure sand 0 0.60 ± 0.04 2.4 ± 0.2 10 0.53 ± 0.06 2.1 ± 0.3 20 0.34 ± 0.11 1.4 ±0. 4 30 0.59 ± 0.10 2.4 ± 0.4 Grass 10 1.47 ± 0.37 5.9 ± 0.9 20 2.23 ± 1.04 8.9 ± 0.7 30 2.02 ± 0.19 8.1 ± 0.6 Moss 40 7.22 ± 0.37 28.9 ± 1.5 Lichenous 40 5.79 ± 0.64 23.2 ± 2.5 Motley-grass 40 6.20 ± 0.27 24.8 ± 1.1 Shrub 40 2.62 ± 1.64 10.5 ± 6.6  V. N. Pereverzev et al. / Natural Science 2 (2010) 600-611 Copyright © 2010 SciRes. OPEN ACCESS increased to 30-50% (Table 8). A distinctive feature of a microbic component of the newly formed soils, generated on nepheline sands, from acid soils of the region on moraine sediments, was the high number of actinomycetes of genus Streptomyces, class Actinobacteria. In forest podzols their amount does not exceed 3.5% of the total number of saprotrophic bacterial block, while in the recultivated tailing dump streptomycetes reach up 25% of the total number of cul- tivated bacteria. The representatives of genus Noca rdia of the same class s were found much less often. Actinomycetes are neutrophils, while water suspen- sions of nepheline sands possess neutral or alkaline reac- tion, at the same time for Al-Fe-humus podzols domi- nating in the Kola Peninsula, acid reaction of the me- dium is characteristic. Actinomycetes produce extracel- lular hydrolases, capable of decomposing complex or- ganic compounds: cellulose, xylogen, chitin, humus sub- stances. As a whole the prokaryotic complex of newly formed soils on nepheline-bearing industrial wastes is presented in Table 9. Its composition includes mainly actinobacteria of gen- era Arthrobacter, Rhodococcus, Micrococcus and Strep- tomyces, adapted for life in oligotrophic media as a res- ult of economical consumption of both exogenous and endogenous substrates. The composition of prokaryotic complex of pure non-recultivated sand and the sand prior to 30-years old, which has overgrown with grasses and mosses, includes mainly actinobacteria of genera Arthr- obacter and Micrococcus, often forming associative col- onies on nutrient media (therefore, their share in the total complex of saprotrophic bacteria could exceed 100%). Table 8. The share of gram-negative bacteria in nepheline sa- nds (% of the total number of saprotrophic bacteria). Sample, plant group “Age” of sand, years Share of Gr - bacteria Pure sand 0 4 10 5 20 10 30 4 Grass 10 21 20 12 30 11 Moss 40 41 Lichen 40 31 Motley-grass 40 52 Shrub 40 39 Table 9. Prokariotic complex of nepheline sands of different period exposition (% from organotrophic bacterial block). Sample, plant group Total number, ×106 cells/g Arthtobacter Chryseobacterium Rhodococcus Micrococcus Streptomyces Bacillus Pure sand 1.0 59.6 0.2 12.0 46.8 3.7 0.7 Grass (10–30 years) 4.5 63.8 0.9 0 32.8 8.4 1.1 Moss (10–30 years) 2.2 69.9 0 0 31.7 4.8 0.9 Moss (40 years) 5.5 20.1 0.8 0 0 25.8 7.4 Lichen (40 years) 6.2 18.0 1.0 0 0 25.8 2.0 Motley-grass (40 years) 10.4 10.0 0.8 0 0 13.5 3.8 Shrubs (40 years) 5.3 10.8 2.4 0 3.4 17.6 2.3 In nepheline sands 5 strains of dominating species of bacteria have been secured with more than 60% of spa- tial frequency of occurrence. Their identification has been carried out using the method of comparative analy- sis of nucleotide sequences of genes, coding 16S rRNA, and their phylogenetic position (“Bioengineering” centre, Moscow) has been determined. Four strains of the iden- tified bacteria have been referred to Actinobacteria class. These are strains of species: Arthrobacter boritoleran, A. ramosus, Rhodococcus fascian, Micrococcus luteus. Actinobacteria are typical dwellers of soils, water, air and are characterized by non-specificity to nutrient sour- ces and can develop in media with the small content of nutrients, thanks to economical consumption of exogen- ous substrates, i.e., they belong to the trophic group of oligotrophic bacteria. Some of them, in particular, actin- omycetes are capable of producing extracellular hydrol- ases and of decomposing complex polymeric compounds. Besides, actinobacteria can develop at very low humidity of substrate and have high adaptive capacities to adverse conditions of the environment in particular they form carotinoids, protecting cells from UV rays. During long-time exposition in the process of forma- tion of newly formed soils on nepheline-bearing indus- trial wastes the structure of prokaryotic complex of mi- crobial communities was changes. The share of gram-ne- gative bacteria increases in them from 4-10% to 30-50%, while the share of actinobacteria, belonging to gram- positive bacteria, including streptomycetes decreases. The domination of gram-negative bacteria in prokaryotic complex and exclusively small content of actinomycetes is characteristic of acid soils of the region on moraine sediments [10]. Based on this we can assume, that evo- lution of microbic community of nepheline sands in process of their recultivation and revegetation follows  V. N. Pereverzev et al. / Natural Science 2 (2010) 600-611 Copyright © 2010 SciRes. OPEN ACCESS the way of rapprochement with microbial communities of zonal soils. 4.3. The Number and Biomass of Fungi The number of microscopic fungi—the basic decompos- ers of organic substance in the recently filled sands from apatite-nepheline manufacture was very small and did not exceed tens of CFU per 1 gram (Figure 3). In the process of increase of the storage period of sands and their revegetation the number of fungi increased to hun- dreds of CFU per gram of substrate. More complete data about the abundance of fungi in soils can be obtained using microscopic counts methods, in particular, the method of fluorescent microscopy. The length of fungal mycelium in nepheline sands, which had been object to recultivation 40 years ago, under lic- hen and motley grass group reached 1000 m/g, and its biomass made 1.3 mg in 1 g of substrate (Table 10). These values are quite comparable to those in soils of taiga forests of the Kola Peninsula [10]. At that, the fu- ngi biomass in the 40-year sands exceeded the bacterial Duration of storage, years Figure 3. Number of microscopical fungi in nepheline sands in the time gradient. Table 10. The length of fungal mycelium (m/g) and the bio- mass of fungi and bacteria (× 10-5 g/g) in nepheline sands. Sample, plant group “Age” of sand, years Length of fungal mycelium Fungal biomass Total biomass of fungi and bacteria Pure sand 0 12 ± 5 1.3 ± 0.6 3.7 ± 0.4 10 32 ± 2 3.5 ± 0.2 5.6 ± 0.2 20 43 ± 13 4.7 ± 1.5 6.1 ± 0.9 30 26 ± 5 2.9 ± 0.5 5.3 ± 0.4 Grass 10 59 ± 8 6.4 ± 0.9 12.3 ± 0.7 20 62 ± 8 6.9 ± 0.8 15.8 ± 0.7 30 112 ± 35 12.4 ± 3.9 20.5 ± 2.2 Moss 40 710 ± 145 78.1 ± 16.0 107.0 ± 8.7 Lichen 40 1156 ± 192 127.2 ± 21.1 150.4 ± 11.8 Motley-grass 40 1064 ± 74 117.0 ± 8.1 141.8 ± 4.6 Shrub 40 434 ± 159 47.8 ± 1.7 58.3 ± 4.1 one 3-5 times which is characteristic of organic horizons of the zone of spruce forests of the Kola North. In the recently filled sands the indices of mycelium length and its biomass were much lower and did not exceed 12 m and 0.013 mg/g respectively. It should be noted, that in sands without vegetation the contribution of bacteria and fungi to the total microbic biomass is equivalent, and in the sands subjected to phytomelioration, the fungi biomass exceeds 10 times that of bacteria. 4.4. Fungi Species Diversity At present we have identified in the sands of the tailing dump, reclaimed over 40 years ago, —26 species related to 10 genera, 7 orders, 4 classes and 2 divisions; in rece- ntly filled sands—only 12 species related to 8 genera, 5 orders, 4 classes and 2 divisions. Most widely represent- ted in the complex of micromycetes of the reclaimed nepheline tailing dump have been fungi of Penicillium genus. They made over 50% of all species diversity of the identified fungi. In recently filled sands the given genus was represented by 4 species, in the recultivation one – by 15 species. In nepheline sands recultvated over 40 years ago, the group of often found fungi included the species: Morti- erella longicollis, Phoma eupyrena, Penicillium daleae. Fungi Acremonium rutilum, Fusarium solani, Mucor hiemalis, M. plumbeus, Penicillium variabile have been identified only in recently filled nepheline sands. These species of fungi have been also found in apatite-nep- heline underground mining workings [11] and in produ- cts of technological conversion at apatite-nepheline con- centrating mills [9], whence they could go to the tailing dump. No dominating species were found in recently filled sands, which are also confirmed by the decrease of the value of Simpson domination index and, respectively, the increase of the value of Pielou evenness index (Ta- ble 11). In recultivated sands Simpson index was equal to 0.26, Pielou—0.53; in recently filled sands to 0.15 and 0.96 Table 11. Some indices of species structure of nepheline sand fungi community. “Age” of sand Shannon total diversity Simpson domin ation Pielou evenness 0 1.99 0.15 0.96 10 1.31 0.4 0.46 20 1.63 0.37 0.53 30 1.57 0.29 0.55 40 1.7 0.26 0.53 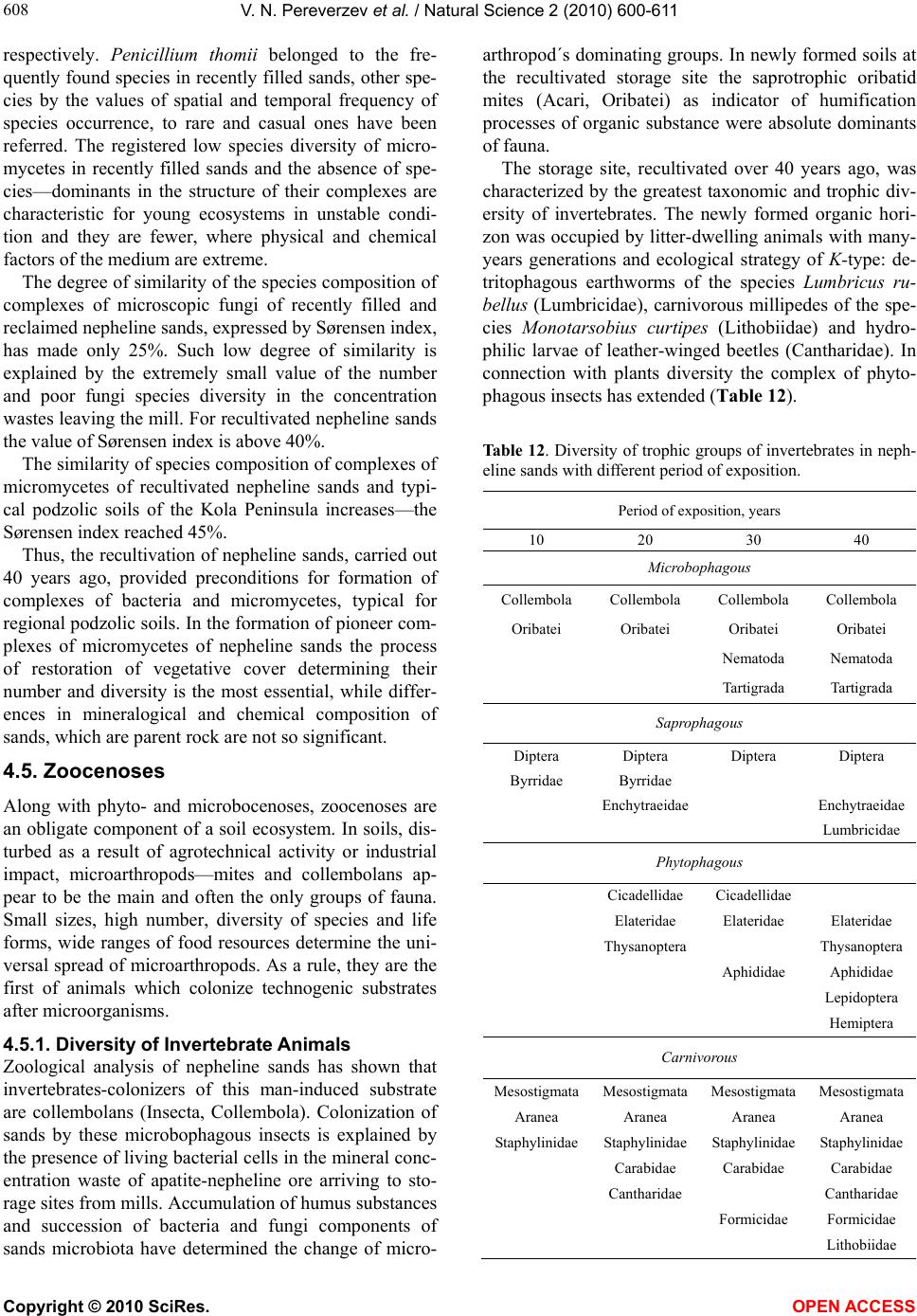 V. N. Pereverzev et al. / Natural Science 2 (2010) 600-611 Copyright © 2010 SciRes. OPEN ACCESS respectively. Penicillium thomii belonged to the fre- quently found species in recently filled sands, other spe- cies by the values of spatial and temporal frequency of species occurrence, to rare and casual ones have been referred. The registered low species diversity of micro- mycetes in recently filled sands and the absence of spe- cies—dominants in the structure of their complexes are characteristic for young ecosystems in unstable condi- tion and they are fewer, where physical and chemical factors of the medium are extreme. The degree of similarity of the species composition of complexes of microscopic fungi of recently filled and reclaimed nepheline sands, expressed by Sørensen index, has made only 25%. Such low degree of similarity is explained by the extremely small value of the number and poor fungi species diversity in the concentration wastes leaving the mill. For recultivated nepheline sands the value of Sørensen index is above 40%. The similarity of species composition of complexes of micromycetes of recultivated nepheline sands and typi- cal podzolic soils of the Kola Peninsula increases—the Sørensen index reached 45%. Thus, the recultivation of nepheline sands, carried out 40 years ago, provided preconditions for formation of complexes of bacteria and micromycetes, typical for regional podzolic soils. In the formation of pioneer com- plexes of micromycetes of nepheline sands the process of restoration of vegetative cover determining their number and diversity is the most essential, while differ- ences in mineralogical and chemical composition of sands, which are parent rock are not so significant. 4.5. Zoocenoses Along with phyto- and microbocenoses, zoocenoses are an obligate component of a soil ecosystem. In soils, dis- turbed as a result of agrotechnical activity or industrial impact, microarthropods—mites and collembolans ap- pear to be the main and often the only groups of fauna. Small sizes, high number, diversity of species and life forms, wide ranges of food resources determine the uni- versal spread of microarthropods. As a rule, they are the first of animals which colonize technogenic substrates after microorganisms. 4.5.1. Diversity of Invertebrate Animals Zoological analysis of nepheline sands has shown that invertebrates-colonizers of this man-induced substrate are collembolans (Insecta, Collembola). Colonization of sands by these microbophagous insects is explained by the presence of living bacterial cells in the mineral conc- entration waste of apatite-nepheline ore arriving to sto- rage sites from mills. Accumulation of humus substances and succession of bacteria and fungi components of sands microbiota have determined the change of micro- arthropod´s dominating groups. In newly formed soils at the recultivated storage site the saprotrophic oribatid mites (Acari, Oribatei) as indicator of humification processes of organic substance were absolute dominants of fauna. The storage site, recultivated over 40 years ago, was characterized by the greatest taxonomic and trophic div- ersity of invertebrates. The newly formed organic hori- zon was occupied by litter-dwelling animals with many- years generations and ecological strategy of K-type: de- tritophagous earthworms of the species Lum bricus ru- bellus (Lumbricidae), carnivorous millipedes of the spe- cies Monotarsobius curtipes (Lithobiidae) and hydro- philic larvae of leather-winged beetles (Cantharidae). In connection with plants diversity the complex of phyto- phagous insects has extended (Table 12). Table 12. Diversity of trophic groups of invertebrates in neph- eline sands with different period of exposition. Period of exposition, years 10 20 30 40 Microbophagous Collembola Collembola Collembola Collembola Oribatei Oribatei Oribatei Oribatei Nematoda Nematoda Tartigrada Tar tigrada Saprophagous Diptera Diptera Diptera Diptera Byrridae Byrrida e Enchytraeidae Enchytraeidae Lumbricidae Phytophagous Cicadellidae Cicadellidae Elateridae Elateridae Elateridae Thysanoptera Thysanoptera Aphididae Aphididae Lepidoptera Hemi ptera Carnivorous Mesostigmata Mesostigmata Mesostigmata Mesostigmata Aranea Aranea Aranea Aranea Staphylinidae Staphylinidae Staphylinidae Staphylinidae Carabidae Carabidae Carabidae Cantharidae Cantharidae Formicidae Formicidae Lithobiidae 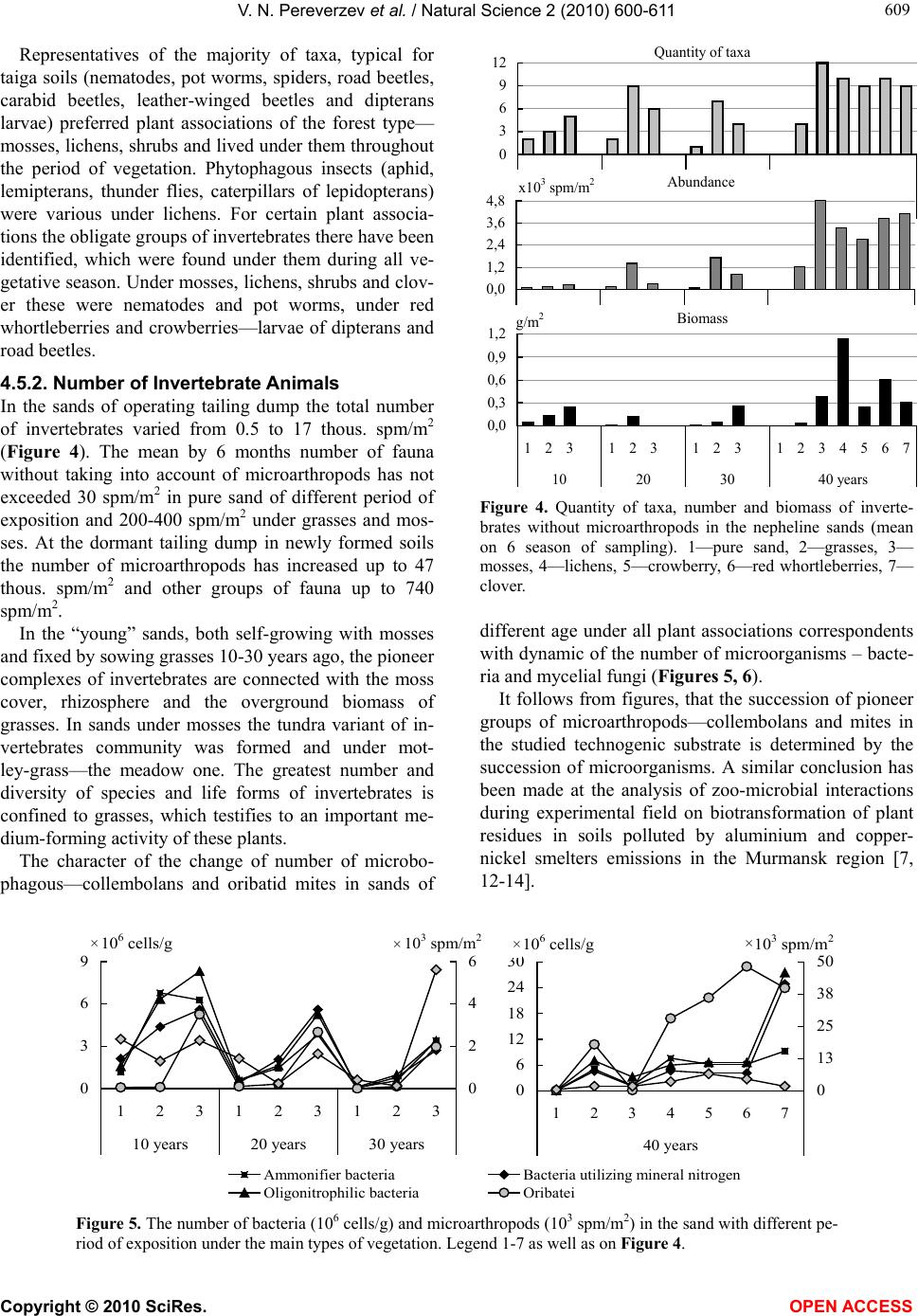 V. N. Pereverzev et al. / Natural Science 2 (2010) 600-611 Copyright © 2010 SciRes. OPEN ACCESS Representatives of the majority of taxa, typical for taiga soils (nematodes, pot worms, spiders, road beetles, carabid beetles, leather-winged beetles and dipterans larvae) preferred plant associations of the forest type— mosses, lichens, shrubs and lived under them throughout the period of vegetation. Phytophagous insects (aphid, lemipterans, thunder flies, caterpillars of lepidopterans) were various under lichens. For certain plant associa- tions the obligate groups of invertebrates there have been identified, which were found under them during all ve- getative season. Under mosses, lichens, shrubs and clov- er these were nematodes and pot worms, under red whortleberries and crowberries—larvae of dipterans and road beetles. 4.5.2. Number of Invertebrate Animals In the sands of operating tailing dump the total number of invertebrates varied from 0.5 to 17 thous. spm/m2 (Figure 4). The mean by 6 months number of fauna without taking into account of microarthropods has not exceeded 30 spm/m2 in pure sand of different period of exposition and 200-400 spm/m2 under grasses and mos- ses. At the dormant tailing dump in newly formed soils the number of microarthropods has increased up to 47 thous. spm/m2 and other groups of fauna up to 740 spm/m2. In the “young” sands, both self-growing with mosses and fixed by sowing grasses 10-30 years ago, the pioneer complexes of invertebrates are connected with the moss cover, rhizosphere and the overground biomass of grasses. In sands under mosses the tundra variant of in- vertebrates community was formed and under mot- ley-grass—the meadow one. The greatest number and diversity of species and life forms of invertebrates is confined to grasses, which testifies to an important me- dium-forming activity of these plants. The character of the change of number of microbo- phagous—collembolans and oribatid mites in sands of 0 3 6 9 12 1231231231234567 10203040 years 0,0 1,2 2,4 3,6 4,8 1 2 31 2 312 312 34 5 6 7 10203040 years 0,0 0,3 0,6 0,9 1,2 123 123 123 1234567 10203040 years Figure 4. Quantity of taxa, number and biomass of inverte- brates without microarthropods in the nepheline sands (mean on 6 season of sampling). 1—pure sand, 2—grasses, 3— mosses, 4—lichens, 5—crowberry, 6—red whortleberries, 7— clover. different age under all plant associations correspondents with dynamic of the number of microorganisms – bacte- ria and mycelial fungi (Fig ures 5, 6). It follows from figures, that the succession of pioneer groups of microarthropo d s—collembolans and mites in the studied technogenic substrate is determined by the succession of microorganisms. A similar conclusion has been made at the analysis of zoo-microbial interactions during experimental field on biotransformation of plant residues in soils polluted by aluminium and copper- nickel smelters emissions in the Murmansk region [7, 12-14]. 0 3 6 9 123123123 10 years20 years30 years 0 2 4 6 Ammonifier bacteriaBacteria utilizing mineral nitrogen Oligonitrophilic bacteriaOribatei 0 6 12 18 24 30 1 2 3 4 5 6 7 40 years Figure 5. The number of bacteria (106 cells/g) and microarthropods (103 spm/m2) in the sand with different pe- riod of exposition under the main types of vegetation. Legend 1-7 as well as on Figure 4.  V. N. Pereverzev et al. / Natural Science 2 (2010) 600-611 Copyright © 2010 SciRes. OPEN ACCESS 0 0,8 1,6 2,4 3,2 123123123 10 years20 years30 years Microfungy Oribatei Collembola 0 50 100 150 200 250 1234567 40 years Figure 6. The number of microfungi (103 CFU/g) and microarthropods (103 spm/m2) in the sands with different period of exposition. Legend 1-7 as well as on Figure 4. Phytomelioration of nepheline sands has positively affected the growth of the number of fauna compared with the sands without vegetation or self-growing with mosses. As a whole, biotic factors have determinant in- fluence on the colonization of this technogenic substrate by various groups of invertebrate animals in comparison with abiotic factors. The bacterial biomass in the newly formed soils on nepheline sands, recultivated over 40 years ago, has increased compared to the pure sand 14 times on the average. The invertebrate’s complex of sands, recultivated over 40 years ago a poor variant of mesofauna of taiga pod- zols of the Kola North with lower taxonomic and trophic diversity and zoomass was represent. Half a century af- ter the carrying out of recultivation of the dormant tail- ing dump, in newly formed soils there was no formation of zoocenoses, characteristic for zonal podzols of the Kola North. 5. CONCLUSIONS A primary soil-forming process is taking place on ore concentration wastes of apatite-nepheline industry, wh- ose biological recultivation was carried out 40 years ago. The manifestation of the soil-forming process in nephe- line sands can be characterized by the following indicat- ions: 1) formation of a thin litter with the content of org- anic carbon at the level of 8-12%; 2) accumulation of humus substances in the mineral sub-litter horizon to the depth of 5 sm as a result of humification of root litter; 3) a distinct change of the response of the medium of the top part (to the depth of 20 sm) of mineral thickness of sands. Microorganisms are biocatalysts of processes of prim- ary soil formation and one of the primary factors, which determine the specificity of this process. In the process of formation of newly formed soils on nepheline-bearing industrial wastes, there occurs a change in the structure of prokaryotic complex of microbial communities, wh- ich originally essentially differed from the prokaryotic complex of zonal soils on moraine sediments. The share of gram-negative bacteria increases in them, while the share of actinobacteria decreases, including streptomy- cetes. In the formation of pioneer complexes of micro- mycetes the process of restoration of the vegetation cov- er, determining their number and diversity, is the most essential, while distinctions in mineralogical and chemi- cal composition of sands, which are parent rock, are not so significant. The recultivation of nepheline sands, car- ried out 40 years ago, provided prerequisites for the formation of complexes of bacteria and micromycetes, typical for regional podzolic soils. Common features of invertebrate’s complexes in nepheline sands with different period of exposition were the low species diversity and the high level of the num- ber of invertebrates; their colonization by small-size and short-living representatives of micro- and mesofauna; dependence of the succession of pioneer groups of mi- croarthropods on the succession of bacteria and fungi. The generated ecosystem as a result of biological re- cultivation and development of the vegetation cover on the surface of nepheline sands represents a natural model of a man-induced formation that underwent a long evo- lution from barren sands, scarcely occupied only by mi- croorganisms, to complex biogeocenoses, which include the vegetation cover of various structures and the newly formed soil. According to modern classification, soils, generated on reclaimed tailings of apatite industry, can be referred to the grey- humus (cespitose) type with AY-C profile of department of organo-accumulative soils of the post-lithogenic soils’s stem [15]. 6. ACKNOWLEDGEMENTS We are grateful to N. Mozgova, N. Voronina, L. Baskova, N. Korobey- nikova and E. Lebedeva for help in analytic work. This work was sup-  V. N. Pereverzev et al. / Natural Science 2 (2010) 600-611 Copyright © 2010 SciRes. OPEN ACCESS ported by Program “Biodiversity” of Presidium of Russian Academy of Sciences. REFERENCES [1] Ginzburg, K.E. (1981) Phosphorus of basic types of soils of the USSR. Nauka, Moscow. [2] Pereverzev, V.N., Koshleva, E.A. and Churikov, A.M. (1992) Phosphorus in podzolic soils of the Kola Penin- sula. Publishing House of the Kola Science Centre RAS, Apatity. [3] Pereverzev, V. N . (1993) Cultural soil formation in the Far North. Publishing House of the Kola Science Centre of the RAS, Apatity. [4] Pereverzev, V. N . , Korobeynikova, N. M. , Dyakova, T.A. and Yanchenko, I . V. (2007) Agrochemical properties and fertility of soils, generated on tailing dumps of apatite industry after their reclamation. Agrochemistry, 1, 5-12. [5] Pereverzev, V. N . and Podlesnay a, N.I. (1986) Biological reclamation industrial dumps in the Far North. Publishing House of the Kola Branch of AS of the USSR, Apatity. [6] Kapelkina, L. P. and Kazakov, L.A. (1989) Wood recla- mation of damaged land in the Polar region. Lesnoye Khozaistvo, 2, 27-29. [7] Evdokimova, G. A ., Zenkova, I . V. , Mozgova, N . P. and Pereverzev, V. N. (2005) Soil and soil biota in the condi- tions of fluorine pollution. Publishing House of the Kola Science Centre RAS, Apatity. [8] Dospekhov, B.A. (1985) The technique of field expe- rience. 5th Edition, Agropromizdat, Мoscow. [9] Gershenkop, A.S., Evdokimova, G. A., Voronina, N . V. and Kreimer, L.L. (2005) Influence of bacterial compo- nent of the recycling water on the flotation of not sul- phidic ores—by the example of “Apatit”. Inzhenernaya Ekologiya, 3, 51-61. [10] Evdokimova, G. A . and Mozgova, N . P. (2001) Microor- ganisms of tundra and forest podzols of the Kola North. Publishing House of the Kola Science Centre of the RAS, Apatity. [11] Evdokimova, G. A. and Naumenko, A.F. (2002) Microor- ganisms of underground mining workings of Northern Fennoscandia. Geoecologya. Inzhenernaya Ekologiya. Gydrogeologya. Geokriologya, 3, 237-242. [12] Evdokimova, G. A . , Zenkova, I .V. and Pereverzev, V. N . (2002) Biodynamics of processes of organic substance transformation in soils of Northern Fennoscandia. Pub- lishing House of the Kola Science Centre RAS, Apatity. [13] Evdokimova, G. A ., Zenkova, I . V. , Mozgova, N . P. and Pereverzev, V. N . (2004) Interactions of soil microorgan- isms and invertebrate animals at the transformation of plant residues in soils of Northern Fennoscandia. Soil Science, 10, 1199-1210. [14] Zenkova, I . V. (2002) Succession changes in communities of invertebrate animals in the course of leaf litter de- composing in the zone of influence of copper-nickel companies. Ecology of Northern Territories of Russia. Problems, Forecast of The Situation, Ways of Develop- ment, Solutions, 2, 371-375. [15] Dobrovolsky, G. V. (2004) Classification and diagnostics of soils in Russia. Oykumena, Smolensk.
|