Paper Menu >>
Journal Menu >>
 Journal of Minerals & Materials Characterization & Engineering, Vol. 11, No.6, pp.597-607, 2012 jmmce.org Printed in the USA. All rights reserved 597 Growth and Characterization of New Non Linear Optical Bis-Glycine Hydro Bromide (BGHB) Single Crystal S. Sampthkrishnana *, N. Balamuruganb, R. Kumuthac, Y. Vidyalakshmid, S. Muthua a Department of Physics , Sri Venkateswara College of Engineering, Sriperumbudur, India. b Department of Physics, PERI Institute of Technology, Chennai-600 048, India. c Department of Physics, JAYA Engineering College, Chennai, India. d Departments of Physics, MIT, Anna University, Chennai-48. *Corresponding Author: Sambathk@svce.ac.in, n_rishibalaa@yahoo.co.in ABSTRACT A new non linear optical material, Bis-Glycine Hydro bromide (BGHB), has been synthesized. Single crystals of BGHB have been grown successfully by slow evaporation method. The solubility of the material was measured in various solvents such as ethanol, acetone and water. It was found to have extremely low solubility in ethanol and acetone. The grown crystals were characterized by recording the powder diffraction and identifying the diffracting planes. Using single crystal diffractometer the morphology of BGHB crystal was identified. Fourier transform infrared (FTIR) spectroscopic studies, optical behavior such as UV-visible-NIR absorption, Thermogravimetic (TG) and differential scanning calorimetric (DSC) analyses have been performed to show that BGHB is thermally stable up to 168.5oC and there is no phase transition and decomposition till 168.5oC. Anisotropy in the hardness behavior has been observed while measuring at different crystal planes by Vicker hardness test. Key words: Solubility, Grown from solution, Crystal structure, FT-IRspectroscopy, Micro hardness, 1. INTRODUCTION In the technological society, development of new devices has been introduced through the growth of single crystals. Crystals for practical and technological applications should have a well developed morphology and contain low density of defects predicted by thermodynamic and  598 S. Sampthkrishnan, N. Balamurugan Vol.11, No.6 kinetic parameters which determine the growth mechanism and the growth kinetics and the generation of defects respectively. This method is extremely popular in the production of many technologically important crystals. Much organic and inorganic crystal can be grown using this technique [1]. Most of the organic NLO crystals usually have poor mechanical and thermal properties and are susceptible for damage during processing even though they have large NLO efficiency. Also, it is difficult to grow larger size optical-quality crystals of these materials for device applications. Purely inorganic NLO materials have excellent mechanical and thermal properties, but possess relatively moderate optical nonlinear property because of the lack of extended π-electron delocalization [2, 3]. L-Cysteine hydrochloride [4], L-Histidine hydrochloride [5], L-Histidine tetrafluroborate [6], L-Histinium bromide [7], L-Histidine hydrofluride dehydrate [8] and L-Glutamic acid hydrocholoride (GHC) [9] are some examples of semiorganic amino acid-related NLO materials reported recently. Presently, the single crystals of a new semiorganic NLO material viz., Bis-Glycine hydrobromide (BGHB) have been grown by slow evaporation method. In the present work, the solubility of BGHB was measured in different solvents. The growth aspects, morphology, the results of the XRPD and FTIR analysis have been discussed. The differential scanning calorimeter (DSC), DifferentialThermalAnalysis (TGA), thermogravimetric (TGA) analysis and SHG efficiency of the powdered materials was measured by using the Kurtz and Perry method [10]. Measurements of microhardness are also reported. 2. EXPERIMENTAL PROCEDURE 2.1 Crystal Growth High purity Glycine salt (E-merck) and hydrobromic acid (E-Merck) were taken in the molar ratio 2:1 in deionized water to synthesis BiGlycine Hydrogen Bromide salt. The pH of the solution was adjusted to be 2 and the growth experiment was maintained at 318K. The saturated BGHB solution of pH 2 has been prepared using doubly recrystallised salt. The solution was filtered using sintered glass filter 1µ porosity. The filtered solution was transferred into the petty disc and allowed to evaporate slowly at room temperature. Transparent and flawless crystals size: (22 x 6 x 8) mm3 were obtained after 10 days as shown in the Figure (1). 2.2 Solubility of BGHB The solubility of BGHB was measured for different solvents (ethanol, methanol and acetone) by gravimetrical analysis method. Water has been selected as a solvent, because the solubility of BGHB is more in water compared with other solvents. The aqueous solution of recrystallised BGHB was prepared and maintained at constant temperature with continuous stirring to ensure homogeneous temperature and concentration over the entire volume of the solution. On reaching saturation, the content of the solution was analyzed gravimetrically. The same procedure was repeated for various temperatures such as 300C, 350C, 400C, 450C and 500C. The plotted 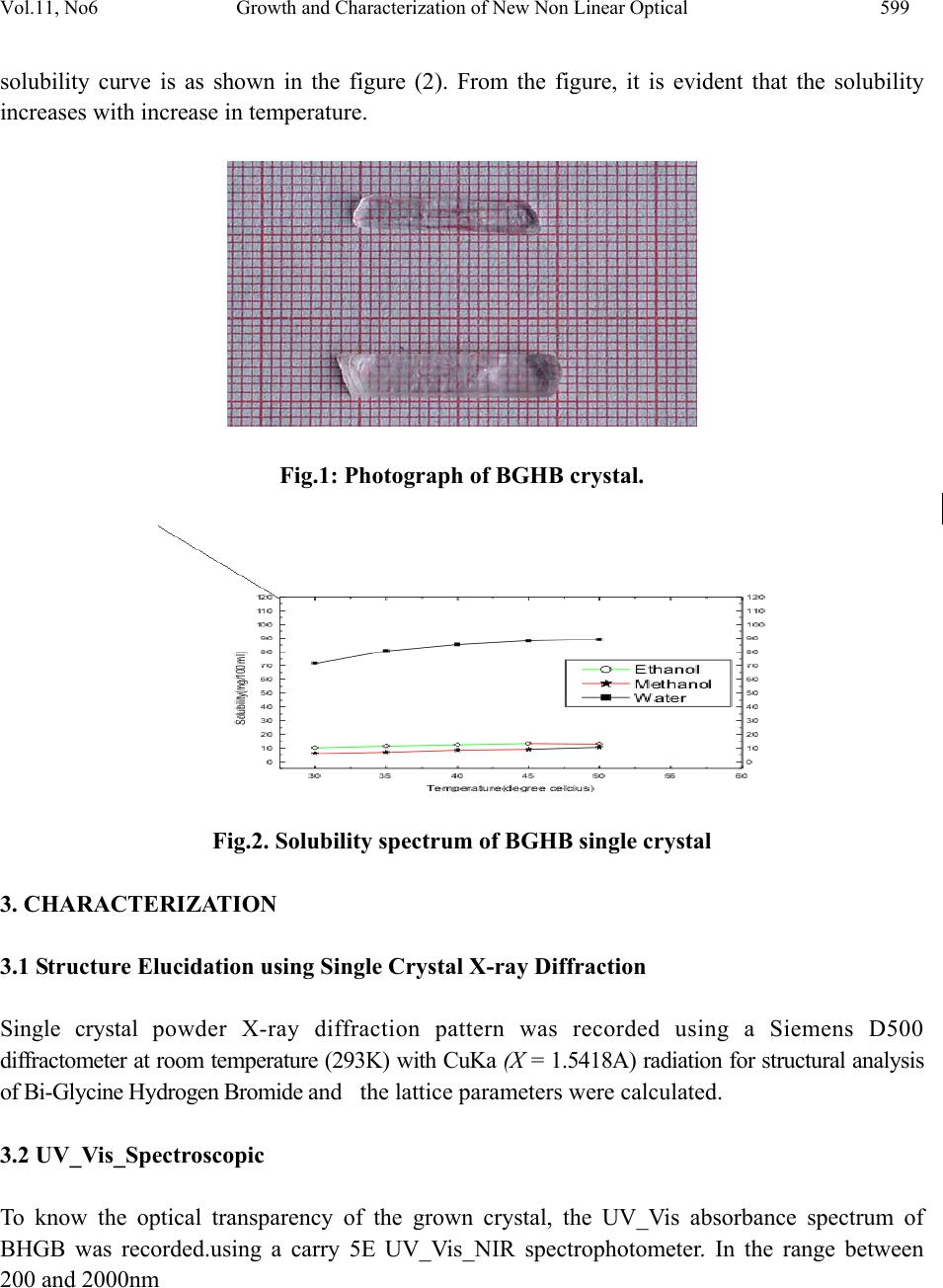 Vol.11, No6 Growth and Characterization of New Non Linear Optical 599 solubility curve is as shown in the figure (2). From the figure, it is evident that the solubility increases with increase in temperature. Fig.1: Photograph of BGHB crystal. Fig.2. Solubility spectrum of BGHB single crystal 3. CHARACTERIZATION 3.1 Structure Elucidation using Single Cry st a l X - r a y D i f f r a c ti o n Single crystal powder X-ray diffraction pattern was recorded using a Siemens D500 diffractometer at room temperature (293K) with CuKa (X = 1.5418A) radiation for structural analysis of Bi-Glycine Hydrogen Bromide and the lattice parameters were calculated. 3.2 UV_Vis_Spectroscopic To know the optical transparency of the grown crystal, the UV_Vis absorbance spectrum of BHGB was recorded.using a carry 5E UV_Vis_NIR spectrophotometer. In the range between 200 and 2000nm  600 S. Sampthkrishnan, N. Balamurugan Vol.11, No.6 3.3 FT-IR Spectroscopic The FTIR spectrum of BGHB crystals was recorded in the range near 2000 - 1800 cm-1by employing a Brukker IFS66 V FTIR spectrometer by KBr pellet technique to study the presence of glycine in the sample qualitatively. 3.4 Microhardness Studies Microhardness measurements were carried out on BGHB crystal using a Leitz Weitzler hardness tester fitted with a diamond pyramidal indentor. The well polished crystal moulted on the platform of microhardness crystal and loads of different magnitudes (5, 10, 20, 30, 40, and 45) were applied over a fixed interval of time. The indentation time was fixed as 5s. 3.5 Thermal Analysis Thermal analysis was used to find out the weight loss (TGA) and energy change (DTA) in the sample with respect to the temperature. The thermal analysis was carried out on the powder specimen of BGHB by employing a MettlerToledoStar simultaneous DTA/TGA analyzer at 10oc/min heating rate in the nitrogen atmosphere by using an alumina crucible. 4. RESULTS AND DISCUSSION 4.1 Crystal Morpholo g y & S ingle Crystal X-ray Structure Analysis Morphology measured using single crystal XRD reveals that there are well- developed faces (102) and their freidels. The indices of the faces are shown in fig (3). In all grown crystals, the most prominent face is (0 1 2). The width of the crystal is in the C direction. To identify the morphology of the grown crystal, the Single crystal XRD was taken and the data were collected using an ENRAF NONIUS CAD4 automatic X-ray diffractometer. The physical crystal had the shape of parallelepiped. The line of intersection of the faces (012) and (01-2) is length of the crystal. The line of intersection is (a*+ b*+ 2c*) x (0a* + b*-2c*) = - 4v*a. Hence it is inferred that the length of crystal is a -direction which is also the shortest unit cell axis of the crystal. (010) and (001) planes are not morphologically developed. The XRD pattern and diffraction indices of the crystal are shown in Fig. 4. The XRPD pattern showed that the synthesized material and the as grown crystals are the single phase of BGHB. The orthorhombic unit cell parameters calculated by TREOR program are a=5.39A0, b=8.18A0, c=18.39A0, α=90.180, β=89.880, γ=89.990 and V=812.4A03, which are comparable with the results determined by a R3m/E four circle X-ray diffractor .  Vol.11, No6 Growth and Characterization of New Non Linear Optical 601 Fig. 3: Morphology of the grown BGHB crystal 10 20 30 40 50 60 70 80 90 0 500 1000 1500 2000 2500 3000 (4 0 5) (3 2 5) (0 4 5) (1 4 3) (2 0 7) (0 0 9) (1 2 6) (0 0 8) (1 3 2) (2 0 3) (1 2 3) (1 2 2)(1 1 4) (1 0 5) (1 0 4)(0 2 2) (0 2 1) (0 0 4) intensity (a r b. units) 2 BGH B Fig. 4: Powder XRD pattern of BGHB single crystal 4.2 UV-Vis –NIR Spectrum The UV-Vis-NIR transmission spectrum (Fig. 5) of the crystal was reorded in the range: 200 – 2000nm using carry 5E UV-Vis_NIR spectrophotometer. It is seen that a strong absorption band occurs at 750 nm and this absorption is due to the n->π* transition. The lower cutoff wavelength occurs at 250 nm & 1600 nm is due to π->π* transition [11]. After that, no absorption takes place in the entire visible region. ( 2 -1 1 ) (0 1 -2) (0 1 2 ( 1 0 0 )  602 S. Sampthkrishnan, N. Balamurugan Vol.11, No.6 Fig.5: UV-vis-NIR spectrum of BGHB single crystal 4.3 FT-IR Studies To identify the elements and the functional groups present in the grown crystal qualitatively, the FTIR spectra were obtained using Brukker IFS66 V FTIR spectrometer by KBr pellet technique. In the FTIR spectrum shown in Figure (6), there is a broad band between 2000 and 3500 cm-1. The sharp peak at 3429, 3113 and 2897 cm-1 may be assigned to NH3+ stretching band. The peaks at 2694 cm-1 and 2601 cm-1 are attributed to the C-H stretching mode vibration. The overtone region contains a band near 2000 - 1800 cm-1 which is assigned to the combination of the asymmetrical NH3+ bending and the torsional oscillation of the NH3+ groups. The intense peaks at 1743, 1715 and 1125 cm-1 indicate the C= O stretching of the COO- group. The asymmetric and symmetric stretching mode of COO- groups are reached by peaks at 1616, 1497, 1446, 1335 and 1254 cm-1. This observation confirms that one glycine is existing in zwitterionic form the peak at 671 cm-1 is due to N-H out-of-plane bending vibration. The torsional oscillation of NH3+ is revealed by the peak 540 cm-1. The strong carbonyl absorption at 1743 cm-1 characterizes α-amino acid hydro bromides. 4000 3000 2000 10000 0 20 40 60 80 100 2199 1829 1545 1290 947 736 3114 1994 1616 1497 1254 886 514 FT-IR BGHB % Transmission W ave number (cm-1) Fig .6.FTIR spectrum of BGHB single crystal  Vol.11, No6 Growth and Characterization of New Non Linear Optical 603 4.4 Micro Hardness Studies Micro hardness measurements were carried out on BGHB crystals. Smooth surface of BGHB was subjected to the Vickers static indentation test at room temperature (303k) using a Leitz Wetzlar hardness tester fitted with a Vickers diamond pyramidal indenter. Loads of different magnitudes (5, 10, 20, 30, 40 and 45g) were applied over a fixed interval of time. The indentation time was kept as 5 s for all the loads. The Vicker micro hardness number was evaluated from the relation Hv = (1.6180 p)/ d2 kg / mm2 (θ = 1080) (Where p is the applied load in kg and d is the diagonal length of the indentation impression in micrometer). 0 102030405060 25 30 35 40 45 50 55 60 65 HV(Kg/mm2) Load P(gram ) (012) Plane (100) Plane Fig. 7: Micro hardness spectrum of BGHB single crystal 4.5 Second Harmonic Generation The Kurtz powder SHG test confirms the NLO property of the grown BGHB crystals [13]. The relative conversion efficiency was calculated from the output power of BGHB crystals with reference to BGHB and KDP crystals output power for various input powers. Table 1 shows the relative conversion efficiency of BGHB crystals for output power. It is observed that the conversion efficiency of BGHB is equal to that of KDP crystals.  604 S. Sampthkrishnan, N. Balamurugan Vol.11, No.6 Table 1: SHG Conversion of KDP, BGHB Output power i n Watt Relative conversion efficiency (SHG in %) of BGHB KDP BGHB 0.6 0.75 125 0.8 0.99 123.5 1 1.16 116 1.2 1.49 125 1.3 1.76 135.4 5. THERMAL STUDIES 5.1 Thermal Gravimetric Analysis In order to find the thermal properties of the grown BGHB crystal, Thermo gravimetric analysis (TGA), and Differential thermal analysis were carried out for BGHB crystal. The TGA were carried out between 25 0C and 1400C in nitrogen atmosphere at a heating rate of 100 C/min using NETZSCH STA 409 C. The fig (8) shows the resulting traces of the grown crystal. 0200 400 600 800100012001400 -20 0 20 40 60 80 100 Weight (%) Temperature (Celcius) Fig.8: TGA Spectrum of BGHB single crystal  Vol.11, No6 Growth and Characterization of New Non Linear Optical 605 5.2 Differential Thermal Analysis In order to find the thermal properties of the grown BGHB crystal, Differential thermal analysis (DTA) were carried out for BGHB crystal. The DTA were carried out between 25 0C and 1400C in nitrogen atmosphere at a heating rate of 100 C/min using NETZSCH STA 409 C. The Fig (9) shows the resulting traces of the grown crystal. 05001000 1500 2000 2500 3000 -1 5 -1 0 -5 0 5 10 mw/mg Temperature (degree celcius) Fig.9: DTA Spectrum of BGHB single crystal 5.3 Differential scanning calorimetry (DSC) In order to find the thermal properties of the grown BGHB crystal, Differential Scanning Calorimetry (DSC) were carried out for BGHB crystal. Show the resulting traces of the grown crystal. The differential scanning Calorimetry (DSC) of BGHB was also carried out between 200C and 2000C at a heating rate of 100C / min in nitrogen atmosphere using NETZSH DSC Z04. It is shown in the Fig (10). 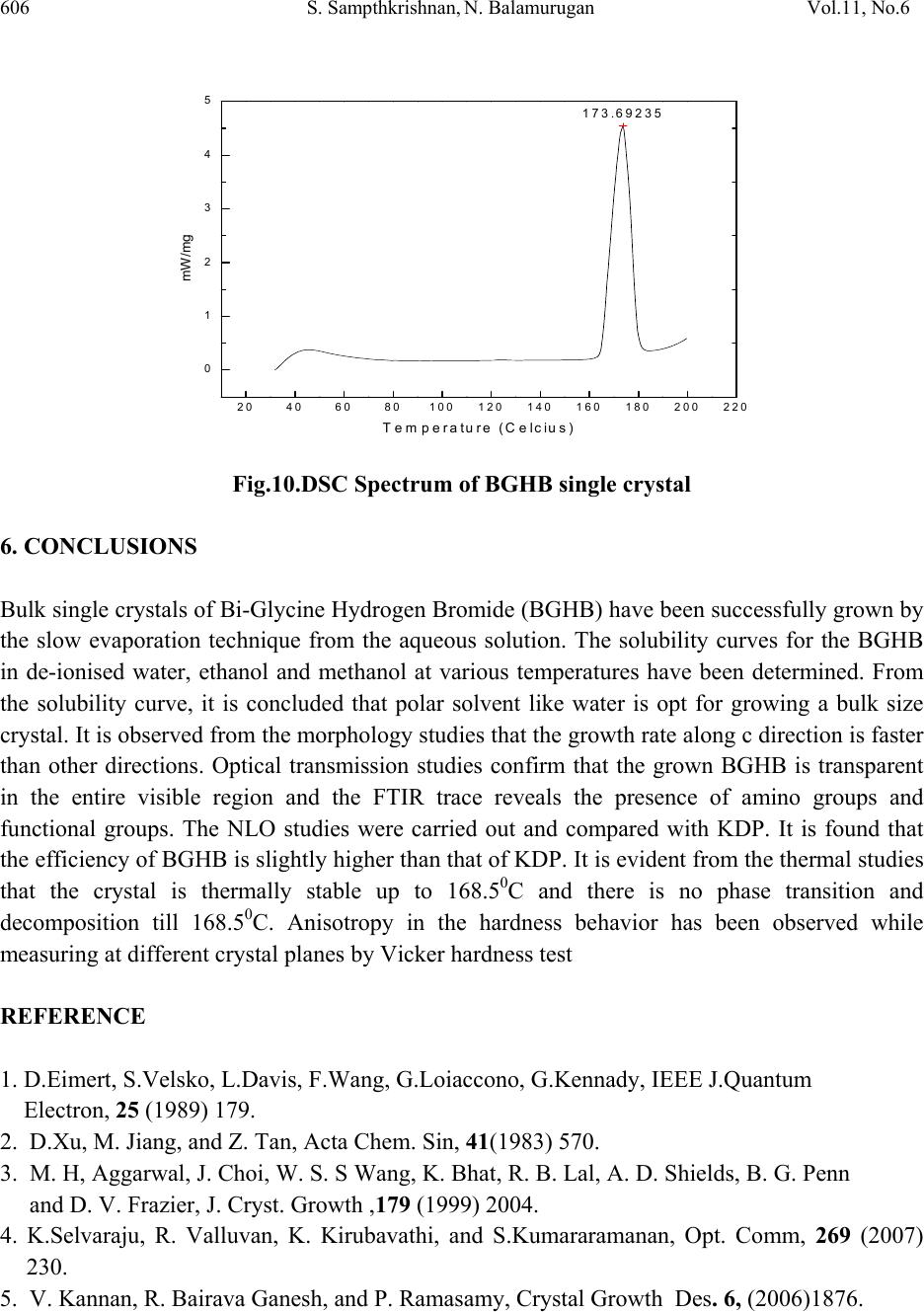 606 S. Sampthkrishnan, N. Balamurugan Vol.11, No.6 20406080100 120 140 160 180 200 220 0 1 2 3 4 5173.69235 mW /mg Temperature (Celcius) Fig.10.DSC Spectrum of BGHB single crystal 6. CONCLUSIONS Bulk single crystals of Bi-Glycine Hydrogen Bromide (BGHB) have been successfully grown by the slow evaporation technique from the aqueous solution. The solubility curves for the BGHB in de-ionised water, ethanol and methanol at various temperatures have been determined. From the solubility curve, it is concluded that polar solvent like water is opt for growing a bulk size crystal. It is observed from the morphology studies that the growth rate along c direction is faster than other directions. Optical transmission studies confirm that the grown BGHB is transparent in the entire visible region and the FTIR trace reveals the presence of amino groups and functional groups. The NLO studies were carried out and compared with KDP. It is found that the efficiency of BGHB is slightly higher than that of KDP. It is evident from the thermal studies that the crystal is thermally stable up to 168.50C and there is no phase transition and decomposition till 168.50C. Anisotropy in the hardness behavior has been observed while measuring at different crystal planes by Vicker hardness test REFERENCE 1. D.Eimert, S.Velsko, L.Davis, F.Wang, G.Loiaccono, G.Kennady, IEEE J.Quantum Electron, 25 (1989) 179. 2. D.Xu, M. Jiang, and Z. Tan, Acta Chem. Sin, 41(1983) 570. 3. M. H, Aggarwal, J. Choi, W. S. S Wang, K. Bhat, R. B. Lal, A. D. Shields, B. G. Penn and D. V. Frazier, J. Cryst. Growth ,179 (1999) 2004. 4. K.Selvaraju, R. Valluvan, K. Kirubavathi, and S.Kumararamanan, Opt. Comm, 269 (2007) 230. 5. V. Kannan, R. Bairava Ganesh, and P. Ramasamy, Crystal Growth Des. 6, (2006)1876.  Vol.11, No6 Growth and Characterization of New Non Linear Optical 607 6. K. V. Rajendran, D. Jayaraman, R.Jayavel, R. Mohan Kumar, and P.Ramasamy, J. Cryst. Growth 224, (2001) 122. 7. K. V.Rajendran, D.Jayaraman, R.Jayavel, P. Ramasamy, J. Cryst. Growth 255, (2003) 361. 8. J.Madhavan, S. Aruna, K. Prabha, J. Packium Julius, p.Joseph Ginson, S.Selvakumar, and S. Sagayaraj, J. Cryst. Growth 293, (2006) 409. 9. K.Selvaraju, R. valluvan, and K. Kumararaman, mater. Let. 60, (2006) 1565. 10. S.K.Kurtz, T.T perry, J. Appl. Phys. 39 (1968) 3798. 11. William Kemp, “Organic Spectroscopy”, Palgrave Macmillan, 1991. 12. R.M. Silverstein, F.X. Webster, Spectrometric Identification of Organic Compounds, 6th Edition, Wiley, New York, 1997 |

