Advances in Bioscience and Biotechnology
Vol.10 No.07(2019), Article ID:94104,9 pages
10.4236/abb.2019.107014
Chemical Composition and Antiviral Effect of Extracts of Origanum vulgare
Daiane Einhardt Blank1, Silvia de Oliveira Hübner2, Gabriela Hörnke Alves3, Claudia Andrea Lima Cardoso4, Rogério Antonio Freitag3, Marlete Brum Cleff2
1Departamento de Química, Universidade Federal de Viçosa, Viçosa, MG, Brazil
2Departamento da Veterinária, Universidade Federal de Pelotas, Pelotas, RS, Brazil
3Center of Chemical, Pharmaceutical and Food Sciences, Universidade Federal de Pelotas, Pelotas, RS, Brazil
4Departamento de Química, Universidade Estadual de Mato Grosso do Sul (UEMS), Dourados, MS, Brazil

Copyright © 2019 by author(s) and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY 4.0).
http://creativecommons.org/licenses/by/4.0/



Received: June 22, 2019; Accepted: July 28, 2019; Published: July 31, 2019
ABSTRACT
This study aimed determine the activity of aqueous and ethanolic extracts of Origanum vulgare against some viruses of veterinary importance (bovine viral diarrhea virus (BVDV), equine arteritis virus (EAV), equine influenza virus (EIV), feline calicivirus (FCV), canine distemper virus (CDV), canine adenovirus (CAV), and canine cororavirus (CCoV) by evaluating the possibility of inhibition of viral particles production. The aqueous extract from 1600 µg/mL did not show cytotoxicity for all cellular lineages evaluated, Madin Darby bovine kidney cells (MDBK), Rabbit kidney cells (RK 13), Madin Darby canine kidney cells (MDCK) and Crandell feline kidney cells (CRFK), and the ethanolic extract of Origanum vulgare was not toxic at 600 µg/mL. The addition of aqueous extract of Origanum vulgare in media resulted in a significant reduction of the EAV titer from 105.42 infecting dose for cellular culture at 50% (TCID50) to 102.09 TCID50/100 µL while in the presence of the ethanolic extract of Origanum vulgare in media resulted in a significant reduction of the EAV titer from 105.42 TCID50 to 100.79 TCID50/100 µL. To CDV the addition of aqueous extract resulted in a reduction from 102.00 TCID50 to 100.00 TCID50/100 µL while in the presence of the ethanolic extract titers were reduced from 102.00 TCID50 to 101.50 TCID50/100 µL. No significant differences in titers regarding the others analyzed viruses were detected. With respect to chemical analysis of the extracts of Origanum vulgare, were identified in the ethanol extract phenolics rosmarinic acid, caffeic acid, carnosol, p-coumaric acid, carnosic acid, luteolin, apigenin, kaempferol and quercetin. In aqueous extracts of Origanum vulgare were detected rosmarinic acid, p-coumaric acid carnosic acid, luteolin, apigenin, kaempferol and quercetin. The data obtained stimulate other biological assays in order to determine which compounds are responsible for the antiviral activity as well as which are the mechanisms involved. The results presented and the considerations we were able to draw from them allowed us to conclude that the ethanolic extract of Origanum vulgare demonstrated lower cell viability than the aqueous extract and has significant antiviral activity against EAV and the both aqueous and ethanolic extracts have antiviral action against CDV.
Keywords:
Virus, Origanum vulgare, Phenolics, Cytotoxicity

1. Introduction
Viral diseases are a challenge for human public health and the farming sector due to their clinical severity, the zoonotic character of some, as well as financial losses. While some diseases are controlled by vaccination programs [1] [2] , others do not have available vaccines in domestic market [3] [4] . The use of antiviral drugs for control and treatment is characterized by several restrictions such as reduced action spectrum, limited therapeutic utility, microbial resistance, high costs, and limited availability [4] [5] . It is difficult to develop new antiviral drugs mainly because of the nature of the viruses, which are totally dependent on cellular metabolic processes to multiply and survive. As a result, the compounds that inhibit viruses or cause their death are also toxic to host cells, at different intensities [5] [6] .
Several researches for the development of new antiviral drugs have been carried out based on synthetic substances. However, great efforts have been made to evaluate the antiviral potential of natural products, in order to isolate and characterize new compounds. That can inhibit viral replication or be used as models of new molecules. Some extracts and essential oils of plants have been evaluated regarding their therapeutic potentiality, and many of them have shown promising biological activities [7] [8] [9] [10] .
Among the various species of plants which are popularly used as an alternative to prevent and treat pathologies, Origanum vulgare stands out due to its reported antibacterial and antifungal properties [11] - [18] . Origanum vulgare is a plant belonging to the family Lamiaceae (Labiatae) [19] . Cognized worldwide as a medicinal, natural antioxidants and flavoring plant, because of bioactive components [20] [21] [22] [23] [24] .
However, it is necessary to evaluate the antiviral potential of this plant, defining its specificity, toxicity and mechanism of action through in vitro and in vivo bioassays, besides identification of the chemical constituents responsible for the biological effects. This work aimed evaluate antiviral activity of ethanolic and aqueous extracts of Origanum vulgare against bovine viral diarrhea virus (BVDV), equine arteritis virus (EAV), equine influenza virus (EIV), feline calicivirus (FCV), canine distemper virus (CDV), canine adenovirus (CAV), and canine cororavirus (CCoV), and identification of the chemical compounds.
2. Materials and Methods
2.1. Acquisition of Extracts
For preparing the aqueous extract 25 g of dry leaves of Origanum vulgare were immersed in 250 mL of distilled water for one hour at 60˚C, under agitation. The extract was then filtered and the process was repeated twice. For obtaining the ethanolic extract, 35 g of dry leaves were added into 350 mL of ethanol for 24 hours at 60˚C, under agitation. After filtering the aqueous and ethanolic extracts, their respective densities were calculated, according to the formula d = m/v. The extracts were then stored in hermetically sealed containers until use.
2.2. Identification of Compounds in the Sample
Chemical constitution was defined through high-performance liquid chromatography (HPLC) by using Varian Diode Array Detector (DAD). The reverse phase column C-18 (Phenomenex Gemini, 25 cm × 4.6 mm × 5 µm) was used for the separation of phenolic compounds. The column was maintained at 40˚C and analyzed for the following wavelengths of interest: 280, 300, and 320 nm. The injection volume was injection volume of 10 µL and the with flow rate of 1 mL∙min−1. The mobile phases were water acidified with phosphoric acid and 1% methanol. Elution of the phenolic compounds was performed using the following gradient mode: 0% - 15% B 2 min; 15% - 25% B for 5 min; 25% - 30% B for 10 min; 30% - 35% B for 15 min; 35% - 50% B for 25 min; 50% - 60% B for 30 min; 60%- 80% B for 35 min; 80% - 100% B for 45 min; and 100% - 5% B for 60 min. The phenolic compounds were identified and quantified based on the analysis of patterns of phenolic compounds (Sigma-Aldrich, >98% purity) under identical analytical conditions used in the samples. The identification parameters applied were spectral similarity, matching the retention times and spectral purity of the peaks to the retention times of interest. The quantification was performed using external standards and 6-point dilution curves (done in triplicate) and a R2 > 0.9 for each individual pattern.
2.3. Viruses and Cells
EAV, EIV, FCV, CDV, CAV, and CCoV were propagated in cell culture as described by Snijder and Meulenberg [25] . The strain Singer of BVDV was propagated in Madin Darby bovine kidney cells (MDBK); EAV (strain Bucyrus) in Rabbit kidney cells (RK 13-ATCC® Number: CCL-37TM); EIV and CDV in Madin Darby canine kidney cells (MDCK), while FCV and CCoV were propagated in Crandell feline kidney cells (CRFK). Viruses culture were frozen when full cytopathic effect (CPE) was observed, after thawing were clarified by centrifugation and the suspensions stored at −70˚C until use. The cells were cultured in Eagle’s medium (E-MEM) containing 10% fetal bovine serum (FBS), and penicillin-streptomycin (Invitrogen, Gaithersberg, MD), at 37˚C and 5% of CO2.
2.4. Cytotoxicity
The aqueous and ethanolic extracts were analyzed regarding toxicity in lineages MDBK, RK13, MDCK, and CRFK by determining the highest non-toxic dilution to be evaluated concerning antiviral action. The cells were cultured in microplates at 37˚C with 5% of CO2 for 24 hours in E-MEM containing 10% of fetal bovine serum, in order to form the monolayers. E-MEM was removed and cells were treated with serial dilutions (in E-MEM) of the aqueous and ethanolic extracts of Origanum vulgare (from 1/10 to 1/5120). Cells treated only with E-MEM were used as control. Cellular viability was measured through MTT assay, after 72 hours, as described [26] . The percentage of viability was calculated as AT/AC × 100; where AT and AC are the rates of absorbance of treated and control cells, respectively.
2.5. Screening for Antiviral Activity
In order to evaluate the presence of antiviral activity, after removing the growth medium of the cellular monolayers, was carried virus titration, in absence or presence of each extract, at a concentration previously determined as non-toxic. Titrations were performed by Behrends & Kärber method [27] , based on absence or presence of cytopathic effect. Reading was done after 72 hours and titer was determined as infecting dose for cellular culture at 50% (TCID50/100 µL).
2.6. Statistical Analyses
All assays described here were performed a minimum of three times and mean values ± SD (Standard Deviation) were calculated using Microsoft Excel®. Statistical analyses were performed using a Fisher’s test and values were considered significant when p < 0.05.
3. Results and Discussion
Extraction resulted in density rates of 1.0002 g/cm3 for the aqueous extract and 0.7846 g/cm3 for the ethanolic extract of Origanum vulgare. From this concentration, serial dilutions were evaluated regarding toxicity in different cell lineages. The determination of cytotoxicity is considered important in order to differentiate cytopathic effect and toxic effect and to select potential vegetal extracts concerning toxicity. The MTT assay showed a decrease in the cellular viability that was proportional to the concentration evaluated in each extract. Absence of cytotoxicity was detected in the aqueous extract at the concentration of 1600 µg/mL, in all lineages used. To the ethanolic extract cellular integrity and viability near to 100% was only observed in the concentration at 600 µg/mL. These concentrations were used in the antiviral assays.
The HPLC-DAD method identified similar phenolic and flavonoid compounds in aqueous and ethanolic extracts of Origanum vulgare. The phenolic acids and diterpenes detected in the ethanolic were carnosic acid, carnosol, p-coumaric acid, rosmarinic acid and caffeic acid. Carnosol and caffeic acid were not detected in the aqueous extract. The flavonoids quercetin, apigenin, luteolin and caempferol were present in both extracts. Chemical constituents here identified were also described in other study carried out with aqueous and ethanolic extracts of Origanum vulgare [28] .
It is known that the solvent used for obtaining the extracts can also interfere in the kinds of chemical constituents, and this may result in different biological activities. Antiviral activity of the plant extracts obtained with different kinds of solvents such as hexane, ethanol and dichloromethane can be different [29] .
Our analytic study was qualitative and the concentrations of the chemical constituents were not determined. Usually the active constituents in bigger concentrations are the responsible by antimicrobial effects.
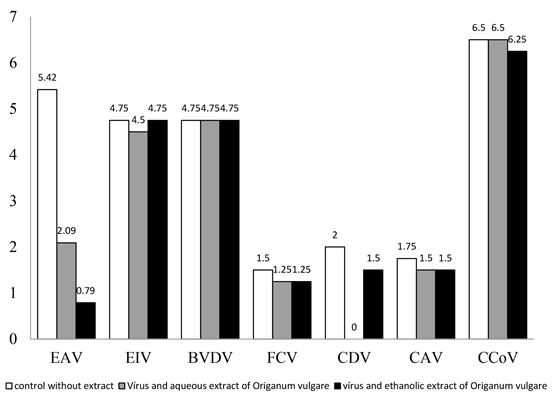
The results of the viruses’ titrations, in absence or presence of each extract, are shown in (Figure 1). The action of the aqueous and ethanolic extract of Origanum vulgare was particularly interesting to EAV, as the virus was almost completely inactivated in the presence of the extract. In the absence of the ethanolic extract EAV showed an average titer of 105.42 TCID50/100 µL and the titer was reduced to 100.79 TCID50/ 100 µL with the presence of these extract, indicating a strong antiviral action. The addition of aqueous extract of Origanum vulgare in media resulted in a significant reduction of the EAV titer from 105.42 TCID50 to 102.09 TCID50/100 µL. When data were statistically analyzed, it was confirmed that EAV titers were significantly different (p < 0.05). Statistically significant results were also obtained to CDV titrated in the presence of the aqueous and ethanolic extracts of Origanum vulgare (Figure 1). The production of particles of BVDV, EIV, FCF, CAV, and CCoV was not significantly affected in the presence of the extracts.
The mechanism of action of the aqueous and ethanolic extracts against EAV and CDV was not determined. It is known that antiviral action can differ to different viruses and even in different stages of infection; the action may be extracellular, in the adsorption to the cells, intracellular, during the replication or also in the stage of liberation of the viral particles [30] .
Moreover, some components described in extracts of Origanum sp. such as flavonoids and phenolic acids can present synergic or antagonistic effect increasing or inhibiting biological potentiality [30] [31] . The synergic effect of the combination of quercertin and baicalein has been demonstrated against human cytomegalovirus (HCMV) [32] .
Among the flavonoids present in the aqueous and ethanolic extracts of Origanum vulgare, quercetin has been described with antiviral activity [33] [34] . It has been suggested that the antiviral potential of quercetin is related to their ability to bind to glycoproteins on the viral envelope and thus interfering in the adsorption and penetration of the virus in the cell and also interfering with DNA synthesis [35] .
It is possible that the compounds quercetin and/or baicalein are related to the antiviral action against EAV and CDV observed in this study. However, in the
Figure 1. Titers (TCID50/100 µL) of EAV, EIV, BVDV, FCV, CDV, CAV and CCoV in absence or presence of aqueous extract (1600 µg/mL) or ethanolic extract (600 µg/mL) of Origanum vulgare.
present work, the antiviral activity against EAV was identified only with the ethanolic extract of Origanum vulgare. This suggests action by compounds absent in the aqueous extract (carnosol and caffeic acid). Regarding CDV, ethanolic and aqueous extract resulted in reduction of production of viral particles. More studies will be carried out aiming to identify the compound or compounds responsible for the anti-EAV and anti-CDV activity and also to determine the mechanism of antiviral action. The results presented in this study give a rich, not yet reported date concerning the antiviral activities of Origanum vulgare, and are therefore a preliminary source of important scientific information that stimulates future investigations.
4. Conclusion
The results presented and the considerations we were able to draw from them allowed us to conclude that the ethanolic extract of Origanum vulgare demonstrated lower cell viability than the aqueous extract and has significant antiviral activity against EAV and aqueous extracts have antiviral action against EAV and both aqueous and ethanolic extracts have antiviral action against CDV.
Conflicts of Interest
The authors declare no conflicts of interest regarding the publication of this paper.
Cite this paper
Blank, D.E., de Oliveira Hübner, S., Alves, G.H., Cardoso, C.A.L., Freitag, R.A. and Cleff, M.B. (2019) Chemical Composition and Antiviral Effect of Extracts of Origanum vulgare. Advances in Bioscience and Biotechnology, 10, 188-196. https://doi.org/10.4236/abb.2019.107014
References
- 1. Meyer, G., Deplanche, M., Roux, D., Moulignie, M., Picard-Hagen, N., Lyazrhi, F., Raboisson, D., Mathevet, P. and Schelcher, F. (2012) Fetal Protection against Bovine Viral Diarrhoea Type 1 Virus Infection after One Administration of a Live-Attenuated Vaccine. The Veterinary Journal, 192, 242-245. https://doi.org/10.1016/j.tvjl.2011.05.011
- 2. Luo, Y., Yuan, Y., Ankenbauer, R.G., Nelson, L.D., Witte, S.B., Jackson, J.A. and Welch, S.W. (2012) Construction of Chimeric Bovine Viral Diarrhea Viruses Containing Glycoprotein E rns of Heterologous Pestiviruses and Evaluation of the Chimeras as Potential Marker Vaccines against BVDV. Vaccine, 30, 3843-3848. https://doi.org/10.1016/j.vaccine.2012.04.016
- 3. Lima, M. and Osorio, F.A. (2007) Arteriviridae. In: Flores, E.F., Eds., Virologia Veterinária, Editora UFSM, Santa Maria/RS, 641-653.
- 4. Yuan, C.Z. and Dwight. C.H. (2003) Microbiologia Veterinária Editora: Guanabara.
- 5. Muller, V., Chavez, J.H., Reginatto, F.H., Zucolotto, S.M., Niero, R., Navarro, D., Yunes, R.A., Schenkel, E.P., Barardi, C.R.M., Zanetti, C.R. and Simoes, C.M.O. (2007) Evaluation of Antiviral Activity of South American Plant Extracts against Herpes Simplex Virus Type I and Rabies Virus. Phytotherapy Research, 21, 970-974. https://doi.org/10.1002/ptr.2198
- 6. Flores, E.F. (2007) Estrutura e composicao dos vírus. In: Flores, E.F., Eds., Virologia Veterinária, Editora UFSM, Santa Maria/RS, 21-33.
- 7. Nolkemper, S., Reichling, J., Stintzing, F.C., Carle, R. and Schnitzler, P. (2006) Antiviral Effect of Aqueous Extracts from Species of the Lamiaceae Family against Herpes Simplex Virus Type 1 and Type 2 in Vitro. Planta Medica, 72, 1378-1382. https://doi.org/10.1055/s-2006-951719
- 8. Bakkali, F., Averbeck, S., Averbeck, D. and Idaomar, M. (2008) Biological Effects of Essential Oils: A Review. Food and Chemical Toxicology, 46, 446-475. https://doi.org/10.1016/j.fct.2007.09.106
- 9. Koch, C., Reichling, J., Kehm, R., Sharaf, M.M., Zentgraf, H., Schneele, J. and Schnitzler, P. (2008) Efficacy of Anise Oil, Dwarf-Pine Oil and Chamomile Oil against Thymidine-Kinase-Positive and Thymidine-Kinase-Negative Herpesviruses. Journal of Pharmacy and Pharmacology, 60, 1545-1550. https://doi.org/10.1211/jpp.60.11.0017
- 10. Saderi, H. and Abbasi, M. (2011) Evaluation of Anti-Adenovirus Activity of Some Plants from Lamiaceae Family Grown in Iran in Cell Culture. African Journal of Biotechnology, 10, 17546-17550.
- 11. Neves, J.M., Matos, C., Moutinho, C., Queiroz, G. and Gomes, L.R. (2009) Ethnopharmacological Notes about Ancient Uses of Medicinal Plants in Trás-os-Montes (Northern of Portugal). Journal of Ethnopharmacology, 124, 270-283. https://doi.org/10.1016/j.jep.2009.04.041
- 12. Cleff, M.B., Meinerz, A.R., Faria, R.O., Xavier, M.O., Santin, R., Nascente, P.S., Rodrigues, M.R. and Meireles, M.C.A. (2010) Atividade inibitória do óleo essencial de orégano em fungos de importancia médica e veterinária. Arquivo Brasileiro de Medicina Veterinária e Zootecnia, 62, 1291-1294. https://doi.org/10.1590/S0102-09352010000500040
- 13. Sahin, F., Gulluce, M., Daferera, D., Sokmen, A., Sokmen, M., Polissiou, M. and Ozer, H. (2004) Biological Activities of the Essential Oils and Methanol Extract of Origanum vulgare ssp. vulgare in the Eastern Anatolia Region of Turkey. Food Control, 15, 549-557. https://doi.org/10.1016/j.foodcont.2003.08.009
- 14. Sarac, N. and Ugur, A. (2008) Antimicrobial Activities of the Essential Oils of Origanum onites L., Origanum vulgare L. Subspecies hirtum (Link) Ietswaart, Satureja thymbra L., and Thymus cilicicus Boiss. & Bal. Growing Wild in Turkey. Journal of Medicinal Food, 11, 568-573. https://doi.org/10.1089/jmf.2007.0520
- 15. Kursat, M., Emre, I., Yilmaz, O. and Erecevit, P. (2011) Antioxidant and Antimicrobial Activity in the Seeds of Origanum vulgare L. Subsp. gracile (C. Koch) Ietswaart and Origanum acutidens (Hand.-Mazz.) Ietswaart from Turkey. Grasas y Aceites, 62, 410-417. https://doi.org/10.3989/gya.113610
- 16. Gulluce, M., Karadayi, M., Guvenalp, Z., Ozbek, H., Arasoglu, T. and Baris, O. (2012) Isolation of Some Active Compounds from Origanum vulgare L. ssp. vulgare and Determination of Their Genotoxic Potentials. Food Chemistry, 130, 248-253. https://doi.org/10.1016/j.foodchem.2011.07.024
- 17. Martins, N., Barros, L., Santos-Buelga, C., Henriques, M., Silva, S. and Ferreira, I.C. (2014) Decoction, Infusion and Hydroalcoholic Extract of Origanum vulgare L.: Different Performances Regarding Bioactivity and Phenolic Compounds. Food Chemistry, 158, 73-80. https://doi.org/10.1016/j.foodchem.2014.02.099
- 18. Bendifallah, L., Tchoulak, Y., Djouabi, M., Oukili, M. and Ghezraoui, R. (2015) Phytochemical Study and Antimicrobial Activity of Origanum vulgare L. (Lamiaceae) in Boumerdes Mountainous Region (Algeria). Journal of Medical and Bioengineering, 4, 471-474. https://doi.org/10.12720/jomb.4.6.471-474
- 19. Singletary, K. (2010) Oregano: Overview of the Literature on Health Benefits. Nutrition Today, 45, 129-138. https://doi.org/10.1097/NT.0b013e3181dec789
- 20. Zhang, X.L., Guo, Y.S., Wang, C.H., Li, G.Q., Xu, J.J., Chung, H.Y., Ye, W.-C., Li, Y.-L. and Wang, G.C. (2014) Phenolic Compounds from Origanum vulgare and Their Antioxidant and Antiviral Activities. Food Chemistry, 152, 300-306. https://doi.org/10.1016/j.foodchem.2013.11.153
- 21. Yan, F., Azizi, A., Janke, S., Schwarz, M., Zeller, S. and Honermeier, B. (2016) Antioxidant Capacity Variation in the Oregano (Origanum vulgare L.) Collection of the German National Genebank. Industrial Crops and Products, 92, 19-25. https://doi.org/10.1016/j.indcrop.2016.07.038
- 22. Morshedloo, M.R., Craker, L.E., Salami, A., Nazeri, V., Sang, H. and Maggi, F. (2017) Effect of Prolonged Water Stress on Essential Oil Content, Compositions and Gene Expression Patterns of Mono- and Sesquiterpene Synthesis in Two Oregano (Origanum vulgare L.) Subspecies. Plant Physiology and Biochemistry, 111, 119-128. https://doi.org/10.1016/j.plaphy.2016.11.023
- 23. Skerget, M., Kotnik, P., Hadolin, M., Hras, A.R., Simonic, M. and Knez, Z. (2005) Phenols, Proanthocyanidins, Flavones and Flavonols in Some Plant Materials and Their Antioxidant Activities. Food Chemistry, 89, 191-198. https://doi.org/10.1016/j.foodchem.2004.02.025
- 24. Mueller, M., Lukas, B., Novak, J., Simoncini, T., Genazzani, A.R. and Jungbauer, A. (2008) Oregano: A Source for Peroxisome Proliferator-Activated Receptor Antagonists. Journal of Agricultural and Food Chemistry, 56, 11621-11630. https://doi.org/10.1021/jf802298w
- 25. Snijder, E.J. and Meulenberg, J.J. (1998) The Molecular Biology of Arteriviruses. Journal of General Virology, 79, 961-979. https://doi.org/10.1099/0022-1317-79-5-961
- 26. Mosmann, T. (1983) Rapid Colorimetric Assay for Cellular Growth and Survival. Journal of Immunological Methods, 65, 55-63. https://doi.org/10.1016/0022-1759(83)90303-4
- 27. Mayr, A., Bachmann, P.A., Bibrack, B.M. and Wittmann, G. (1982) Virologische Arbeitsmethoden-Band IV-Sicherheit bei virologischen arbeiten-Biometrische Methoden. Gustav Fischer Verlag, Stutgart.
- 28. Danila, O.A., Gatea, F. and Radu, G.L. (2011) Polyphenol Composition and Antioxidant Activity of Selected Medicinal Herbs. Chemistry of Natural Compounds, 47, 22-26. https://doi.org/10.1007/s10600-011-9822-7
- 29. Tafuri, N.F. (2011) Atividade antiviral de extratos vegetais e flavonoides contra o Bovine herpesvirus 1 (BoHV1). Dissertacao (Mestrado em Bioquímica agrícola) Universidade de Vicosa, Minas Gerais.
- 30. Schnitzler, P., Nolkemper, S., Stintzing, F.C. and Reichling, J. (2008) Comparative in Vitro Study on the Anti-Herpetic Effect of Phytochemically Characterized Aqueous and Ethanolic Extracts of Salvia officinalis Grown at Two Different Locations. Phytomedicine, 15, 62-70. https://doi.org/10.1016/j.phymed.2007.11.013
- 31. Khan, M.T.H., Ather, A., Thompson, K.D. and Gambari, R. (2005) Extracts and Molecules from Medicinal Plants against Herpes Simplex Viruses. Antiviral Research, 67, 107-119. https://doi.org/10.1016/j.antiviral.2005.05.002
- 32. Cotin, S., Calliste, C., Mazeron, M., Hantz, S., Duroux, J., Rawlinson, W., Ploy, M. and Alain, S. (2012) Eight Flavonoids and Their Potential as Inhibitors of Human Cytomegalovirus Replication. Antiviral Research, 96, 181-1866. https://doi.org/10.1016/j.antiviral.2012.09.010
- 33. Wang, J.N., Hou, C.Y., Liu, Y.L., Lin, L.Z., Gil, R.R. and Cordell, G.A. (1994) Swertifrancheside, an HIV-Reverse Transcriptase Inhibitor and the First Flavone-Xanthone Dimmer, from Swertia Franchetiana. Journal of Natural products, 57, 211-217. https://doi.org/10.1021/np50104a003
- 34. Sy, L., Rhim, J.Y. and Park, W.B. (2005) Antiherpetic Activities of Flavonoids against Herpes Simplex Virus Type 1(HSV-1) and Type 2 (HSV-2) in Vitro. Archives of Pharmacology Research, 28, 1293-1301. https://doi.org/10.1007/BF02978215
- 35. Formica, J.V. and Regelson, W. (1995) Review of Biology of Quercetin and Related Bioflavanoids. Food Chemical Toxicology, 33, 1061-1080. https://doi.org/10.1016/0278-6915(95)00077-1