Open Journal of Urology
Vol.07 No.03(2017), Article ID:75262,11 pages
10.4236/oju.2017.73008
The Aging Bladder in Females Evaluated by Urodynamics
Moacir Cavalcante de Albuquerque Neto1*, Leslie Clifford Noronha Araujo1, Thome Decio Pinheiro Barros Junior1, Joao Luiz Amaro2, Flavia Cristina Morone Pinto3, Fabio de Oliveira Vilar1, Salvador Vilar Correia Lima1
1Urologic Department, Clinical Hospital, Federal University of Pernambuco, UFPE, Recife, Brazil
2Urologic Service, Botucatu Medical School, Paulista State University, UNESP, Botucatu, Brazil
3Postgraduate Program in Surgery, Department of Surgery, Center for Health Sciences, Federal University of Pernambuco, UFPE, Recife, Brazil

Copyright © 2017 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY 4.0).
http://creativecommons.org/licenses/by/4.0/



Received: February 20, 2017; Accepted: March 28, 2017; Published: March 31, 2017
ABSTRACT
Aim: To determine whether bladder functions deteriorate with age. Methods: Data contained in electronic medical record (INFOMED®) were used in this institutional retrospective review. Analysis was done on the urodynamic studies in women over 18 years old conducted between May 2011 and November 2015. Patients with previous history of pelvic surgery or radiotherapy, neurological disease, vaginal prolapse greater than grade I, congenital urogenital malformations, urinary obstructive disease, diabetes, or the use of any medication that could interfere with bladder function were excluded from the analysis. The urodynamic parameters analyzed were the Maximum Cystometric Capacity (MCC), Voiding Volume (VV), Maximum Flow (Qmax), Bladder Compliance (BC), Detrusor Pressure at Maximum Flow (PdetQmax), Bladder Contractility Index (BCI), Bladder Voiding Efficiency (BVE) and Post-Void Residual Urine Volume (PVR). Patients were further stratified in five groups according to age (A―18 to 40; B―41 to 50; C―51 to 60; D―61 to 70; E―over 70 years old). Results: Out of 3103 urodynamic studies analyzed, 719 were eligible for the study. The average age of patients was 49.3 (+13.2) years old and in all evaluated parameters, statistically significant correlation between age and decline of bladder function was obtained (p < 0.05). Moreover, mathematical equations were presented to estimate the parameters values in relation to age. Conclusions: This study showed a decline in bladder storage function (reduction in MCC and BC) and in bladder emptying function (re- duction in Qmax, PdetQmax, VV, BCI and BVE with an increase in PVR) with age.
Keywords:
Aging, Urinary Bladder Diseases, Urinary Bladder, Neurogenic, Urinary Bladder, Overactive, Urodynamics

1. Introduction
Aging is a normal development process involving structural, functional, and che- mical neurobiological changes [1] . It is a complex process that does not occur simultaneously throughout the body, and it is associated with the presence of a disease [2] [3] [4] . In fact, aging involves multiple endogenous and exogenous factors that must be considered in an integrated way, especially in diagnostic si- tuations [5] . It is estimated that 25% of an individual’s longevity is influenced by genetics, while the rest is influenced by lifestyle and environmental factors [1] [6] .
Due to the fact that there is a worldwide increase in life expectancy, disorders inherent to the elderly population are becoming increasingly frequent in clinical practice [5] . Given the current demographic profile of the elderly population, and the prospect of significant increase in their participation in society, it is important to study age-related diseases, including micturition parameters [7] [8] .
The main micturition changes that occur in elderly women are detrusor overactivity with or without incontinence, stress urinary incontinence (SUI), and detrusor hypocontratility [9] . The latter is a change of major concern because besides impairing quality of life, it can also lead to recurrent urinary tract infections. It leads also to inadequate bladder voiding with the need for clean intermittent catheterization. Deterioration of the upper urinary tract can occur with possible progression to chronic renal failure (CRF) [10] [11] .
This urodynamic study evaluation is in women stratified by age.
2. Materials and Methods
This is a retrospective analysis of urodynamic studies taken from an electronic me- dical record database (INFOMED®), and performed in women at the Department of Urology of our institution between May 2011 and November 2015, after gaining the approval of the Ethics Committee.
For sample homogenization, examinations were stratified according to patient age in five groups: A (18 - 40 years old), B (41 - 50 years old), C (51 - 60 years old), D (61 - 70 years old), and E (women > 71 years). All examinations were conducted in women above 18 years of age. Exclusions were made in those with previous history of pelvic surgery or radiotherapy, neurological disease, vaginal prolapse greater than grade I by ICS classification, congenital urogenital malfor- mations, urinary obstructive disease, diabetes, or in case of use of any medication that could interfere with bladder function as alpha blockers, antimuscarinics, diuretics, anti-depressants, sedatives, anti-anxiolytics and opioids [12] .
The urodynamic parameters analyzed were maximum cystometric capacity (MCC), voiding volume (VV), maximum flow (Qmax), bladder compliance (BC), detrusor pressure at maximum flow (PdetQmax), bladder contractility index (B- CI), bladder voiding efficiency (BVE), and post-void residual urine volume (P- VR).
Quantitative variables were compared between groups through the ANOVA test, followed by Tukey’s post hoc test. Next, the hypothesis that correlations were statistically significant was tested using Pearson’s correlation. Linear regression was also used to evaluate cause-effect relationships and to develop mathematical equations for estimating urodynamic parameters according to age. All statistical calculations were performed using the GraphPad Prism 4® software.
3. Results
A total of 3103 urodynamic studies, conducted between May 2011 and November 2015 and registered in INFOMED® (www.infomed.net.br) electronic medical record, were evaluated. Of these, 719 exams were selected according to the afore- mentioned inclusion and exclusion criteria. Patient ages ranged from 18 to 91 years, with an average of 49.3 ± 13.2 years. The distribution of examinations per age group was 188 (A); 228 (B); 162 (C); 88 (D); and 53 (E). The results are described in Table 1.
Pearson’s correlation test was followed by the Student’s t-test to test for statistical significance and by linear regression between age and the observed parameters. The results for these parameters are shown in Table 2.
With linear regression, statistical significance was obtained between age and the observed parameters. Based on that, the following equations were formulated to obtain standard parameters values according to age:
o MCC = 404.3 − 0.76 × Age,
o VV = 398.5 − 1.42 × Age,
o BC = 16.8 + 0.14 × Age,
o Qmax = 21.2 − 0.07 × Age,
o PdetQmax = 39 − 0.2 × Age,
o PVR = 5.8 + 0.67 × Age,
o BCI = 145 − 0.56 × Age,
o BVE = 102 − 0.27 × Age.
4. Discussion
The present study showed significant results for understanding bladder functions during aging in women. The first noteworthy point is the sample size. Our study comprised of 719 women who underwent urodynamic study at a single health center due to various clinical indications. We used rigorous exclusion criteria to eliminate any factors that might influence bladder function other than age. Only one study used an exclusion criteria similar to ours, but its sample size was smaller (n = 85) and only 14 women were 70 years of age or older. Other studies did not exclude patients with diabetes mellitus or those taking antidepressant medications, both of which are known to influence bladder functions. It is relevant to mention the study by Zimmern et al. (2014), who had a larger
Table 1. Urodynamic parameters: maximum cystometric capacity (MCC), voiding Volume (VV), maximum flow (Qmax) bladder compliance (BC), maximum detrusor pressure flow (PdetQmax), post-void residual urine volume (PVR), bladder contractility index (BCI) and bladder voiding efficiency (BVE). Groups: A (up until 40 years old), B (41 - 50 years old), C (51 - 60 years old), D (61 - 70 years old) and E (above 71 years old). *ANOVA followed by the Tukey post-test. Statistically Significant if p < 0.05. Differences are indicated as follows: a. A ≠ B; b. A ≠ C; c. A ≠ D; d. A ≠ E; e. B ≠ C; f. B ≠ D; g. B ≠ E; h. C ≠ D; j. C ≠ E; h. D ≠ E.
sample than ours (n = 945) but was one of the studies that did not exclude patients with diabetes mellitus; also, they only evaluated women with SUI (Table 3) [13] [14] [15] .
Our study demonstrated that there are alterations in bladder function with age, with significant correlation for all the evaluated parameters. Figure 1 re- presents all parameters described below.
Maximum cystometric capacity
Pfisterer et al. (2006) did not observe a decrease in MCC with age. The MCC in their study was 522 mL for patients older than 60 years, while in patients between 20 and 39 years old, it was 493 ml. Also, they proposed that the loss of
Table 2. Pearson’s correlation test (r) and p-value according to the parameters evaluated: MCC, maximum cystometric capacity; VV, voiding volume; Qmax, maximum flow; BC, bladder compliance; PdetQmax, detrusor pressure at maximum flow; BCI, bladder con- tractility index; BVE, bladder voiding efficiency; PVR, post-void residual urine volume; r, pearson’s correlation test; p, statistical significance.
Table 3. Studies published in the literature and consulted for the present research: UTI, urinary tract infection; IPSS, international prostate symptom score; DM, diabetes mellitus; USG, ultrasonography; SUI, stress urinary infection; UDC, uninhibited detrusor contractions; SCI, spinal cord injury; CNS, central nervous system; PVR, post-void residual urine volume.
Figure 1. (a) MCC and VV; (b) BC and PdetQmax; (c) Qmax and PVR; (d) BCI and BVE.
MCC is due to detrusor hyperactivity and not to aging itself. Likewise, two other studies found no significant differences in this parameter:
Zimmern et al. (2014) reported an average MCC of 375.6 ml and 371.8 ml for patients younger and older than 65 years. Respectively; and Shin, On and Kim (2015) found a mean MCC of 354.2 ml and 361.4 ml for patients younger than 50 years and older than 69, respectively [14] [15] [16] .
Our study found a decrease in MCC in elderly women. The group of patients between 41 and 50 years old (B) had the highest mean MCC (394.3 ml), with a progressive decrease according to age group. For patients older than 71 years (E), the mean MCC was 295 ml, which represents a decrease of almost 100 ml in this parameter (p < 0.001).
Void volume
We found an average void volume of 355.3 mL for patients aged between 41 and 50 years (B) and 225.4 mL for patients over 70 years (E). This behavior is similar to the pattern observed for MCC, demonstrating a decrease in storage capacity with aging. Only one study found statistical significance in this parameter, in agreement with our results. Shin, On and Kim (2015) showed a void volume of 314 mL for patients younger than 50 years and 261.4 mL for patients older than 69 years. Yang and Huang (2003) described a volume of 303 mL for patients younger than 30 years and 239 mL for patients older 70 years, without significant difference. Zimmern et al. (2014) published another study in which this parameter showed no significant differences between age groups, reporting a VV of 310.9 mL and 294.4 mL for patients younger and older than 65 years, respectively [15] [16] [17] .
Bladder compliance
The present study showed BC values of 29.2 mL/cmH2O in the age group 61 - 70 years (D) and 18.9 mL/cmH2O in the age group over 70 years (E), indicating that compliance decreases only after 60 years of age, with a statistically significant negative correlation (r −18, p = 0.032). However, none of the groups presented average BC values below 10 - 12.5 mL/cm H2O, which represents a higher risk of upper urinary tract deterioration. Only one study evaluated the variation in BC with age and the result was not significant. Zimmern et al. (2014) showed BC of 56.5 mL/cm H2O and 58.5 mL/cm H2O in groups of patients younger and older than 65 years, respectively [15] [18] [19] .
Detrusor pressure at maximum flow
In our study, patients younger than 40 years (A) showed a PdetQmax of 31.9 cm H2O, while those older than 70 years (E) showed a PdetQmax of 22.9 cm H2O. Pfisterer et al. found a decrease of 36 cmH2O in 20 - 39-year-old patients to 26 cm H2O in patients older than 60 years, suggesting a decline in voiding function.
Two other studies reported significant difference for this parameter between age groups: Zimmern et al. (2014) showed an PdetQmax of 19.5 cm H2O for patients younger than 65 years and 14.0 cm H2O for patients older than 65 years (p = 0.003). Shin, On and Kim (2015) found an average value of 33 cm H2O for patients younger than 50 years and 26.2 cm H2O for patients older than 69 years (p = 0.016). Conversely, Yang and Huang (2003) found no statistical difference for this parameter between age groups [14] [15] [16] [19] .
Post-void residual volume
PVR is a parameter used to evaluate the void functionality of the bladder. We found a significant difference between PVR values of different age groups: 31.9 mL for patients younger than 40 years (A) and 69.6 mL for patients older than 70 years (E). In the Yang and Huang (2003) study, PVR was the only parameter with statistical significant difference (p = 0.003), with 37 mL for patients younger than 30 years; 17 mL for patients between 30 and 70 years old; and 57 mL for patients older than 70 years. Shin, On, and Kim (2015) also found statistical significance between age groups (160.1 mL and 120.2 mL, p = 0.0042). Conversely, Zimmern et al. (2014) and Pfisterer et al. (2006) failed to demonstrate alterations in PVR with aging [11] [13] [14] [19] .
Maximum flow
The Qmax was another parameter that was evaluated. There is a difference with statistical significance among the groups. The values obtained were extracted from the flowmetry from the flow-pressure study and not from the free flowmetry. It was found 18.6 ml/s in the group 41 - 50 years and 14.5 ml/s in the group older than 70 years.
Pfisterer et al. (2006) evaluated both the Qmax in the free flowmetry and in the flow-pressure study, finding statistical significance only in the second. In the group from 20 to 39 years old, the mean Qmax was 26 ml/s and in the group aged 60 years or more, 20 ml/s (p = 0.006). (13) Two other studies found statistical difference. Zimmern et al. (2014) and Shin, On and Kim (2015) obtained a Qmax of 26.2 ml/s (<65 years) and 22.0 ml/s (>65 years) with p = 0.002 and 26.3 ml/s (<50 years) and 22.9 ml/s (>69 years) with p < 0.001, respectively. These findings were obtained by free flowmetry [15] [16] . However, Yang and Huang (2003) did not find statistical significance. The mean Qmax was 21 ml/s (group < 30 years) and 18 ml/s (group > 70 years) [17] .
Bladder contractility index
Developed by Abrams, BCI is a numeric parameter to evaluate bladder function that is more appropriate than the nomograms for statistical and research purposes [19] .
In the present study, we observed a significant difference between BCI values of different age groups, with 123.4 and 95.95 for patients aged between 41 and 50 years (B) and over 71 years (E). Based on this result, we show that there is a decline in bladder function in elderly individuals, since patients with BCI lower than 100 are classified as positive for weak detrusor contraction.
Two other studies evaluated BCI variation with age. Shin, On, and Kim (2015) reported BCI values of 160.1 and 120.2 for patients younger than 50 and older than 69 years old (p = 0.046). Conversely, Zimmern et al. (2014) failed to demonstrate significant difference between BCI of patients younger (BCI = 125.5) and over (BCI = 115.0) 65 years, obtaining a p value of 0.12. However, when the authors evaluated this parameter continuously, they reported that BCI decreased 7.68 ± 1.96 for every 10 years of age increase (p < 0.001) [15] [16] .
Bladder voiding efficiency
BVE was also developed by Abrams, although it is much less used than BCI. This index evaluated the degree of bladder emptying [20] .
Our results showed BVE values of 91.1 and 75.5 for patients aged 51 - 60 years (C) and those aged > 70 years (E), respectively. These values indicate a clear decrease in bladder voiding capacity in more advanced age groups, presenting a graph curve similar to BCI.
We did not find the use of BVE as an evaluation parameter of bladder function in other studies. Nevertheless, in the present study, this parameter behaved similarly to BCI and was compatible with the other parameters.
Estimation of urodynamic parameters according to age
Linear regression is a statistical tool used in situations where a possible cause- and-effect relationship between two quantitative variables exists. In our case, aging would be the cause and bladder function decline would be the effect. The expression of results in this type of statistical inference aims to evaluate the dependence of x in relation to y, and to express this relation mathematically using the following formula:
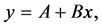
where y is the dependent variable (response), x is the independent variable (predictive), A is a linear coefficient, and B is the angular coefficient.
Hence, we propose equations that allow the estimation of urodynamic parameters values according to age. Formulas are routinely used to estimate the expected MCC in children, but we only found one report using this estimate in adults. As in our study, equations were established through regression. We found no other publication that gives estimates of the other parameters according to age [21] .
Study limitations
It is a retrospective data analysis study; it may be subjected to bias and data loss. Another limitation was the evaluation of Qmax, which in our study was obtained by the flow pressure study and not by free flowmetry. Pfisterer et al. (2007) found a difference between the values. In the flow pressure study, the mean Qmax was 22 ml/s and in the free flowmetry were 26 ml/s [22] .
Moreover, in order to have a precise evaluation of the evolution of bladder function with age in an ideal scientific scenario, it would be necessary to perform urodynamic studies in the same individual throughout life. However, ethical and moral issues would prevent a study with this proposal, since it is an invasive and uncomfortable examination for the patient.
Decline in bladder function
Theories have been proposed to explain the decline of bladder function with age. Pfisterer (2006) proposed that the sarcopenia of the elderly could affect the bladder muscles, with menopause having an important role in the etiology [17] [23] [24] . Animal studies suggest that structural alterations and sensory innervation of the bladder are responsible for bladder changes due to aging [25] [26] . Other factor, the parity, also influences the bladder function [27] .
5. Conclusion
This study showed a decline in bladder storage function (reduction in MCC and BC) and in bladder emptying (reduction in Qmax, PdetQmax, VV, BCI and BVE with an increase in PVR) with age.
Cite this paper
de Albuquerque Neto, M.C., Araujo, L.C.N., Barros Jr., T.D.P., Amaro, J.L., Pinto, F.C.M., de Oliveira Vilar, F. and Lima, S.V.C. (2017) The Aging Bladder in Females Evaluated by Urodynamics. Open Journal of Urology, 7, 54-64. https://doi.org/10.4236/oju.2017.73008
References
- 1. Dos Santos, F., Andrade, V.M. and Bueno, O.F.A. (2009) Aging: A Multifatorial Process. Psicologia em estudo, 14, 3-10.
https://doi.org/10.1590/S1413-73722009000100002 - 2. Kenny, G.P., Groeller, H. and McGinn, R. (2016) Age, Human Performance, and Physical Employment Standards. Applied Physiology, Nutrition, and Metabolism, 41, 92-107.
https://doi.org/10.1139/apnm-2015-0483 - 3. Biehl, M., Takahashi, P.Y. and Cha, S.S. (2016) Prediction of Critical Illness in Elderly Outpatients Using Elder Risk Assessment: A Population-Based Study. Clinical Interventions in Aging, 11, 829-834.
- 4. Baumann, C.W., Kwak, D. and Liu, H.M. (2016) Age-Induced Oxidative Stress: How Does It Influence Skeletal Muscle Quantity and Quality? Journal of Applied Physiology, 121, 1047-1052.
https://doi.org/10.1152/japplphysiol.00321.2016 - 5. Steptoe, A., Deaton, A. and Stone, A.A. (2015) Subjective Wellbeing, Health, and Ageing. The Lancet, 385, 640-648.
https://doi.org/10.1016/S0140-6736(13)61489-0 - 6. Greenwood, H. and Bartlett, D.B. (2013) Meeting Report: British Society for Research on Ageing (BRSA). Annual Scientific Meeting 2012, Vol. 2, Birmingham, 3-4 July 2012, 6.
https://doi.org/10.1186/2046-2395-2-6 - 7. Araki, I., Zakoji, H. and Komuro, M. (2003) Lower Urinary Tract Symptoms in Men and Women without Underlying Disease Causing Micturition Disorder: A Cross-Sectional Study Assessing the Natural History of Bladder Function. The Journal of Urology, 170, 1901-1904.
https://doi.org/10.1097/01.ju.0000092942.87643.27 - 8. Madersbacher, S., Pycha, A. and Kringler, C.H. (1999) Interrelationships of Bladder Compliance with Age, Detrusor Instability, and Obstruction in Elderly Men with Lower Urinary Tract Symptoms. Neurourology and Urodynamics, 18, 3-15.
https://doi.org/10.1002/(SICI)1520-6777(1999)18:1<3::AID-NAU2>3.0.CO;2-4 - 9. Aggarwal, H. and Zimmern, P.E. (2016) Underactive Bladder. Current Urology Reports, 17, 17.
https://doi.org/10.1007/s11934-016-0582-6 - 10. Lachowsky, M. and Nappi, R.E. (2009) The Effects of Estrogen on Urogenital Health. Maturitas, 63, 149-151.
https://doi.org/10.1016/j.maturitas.2009.03.012 - 11. Nelson, H.D. (2008) Menopause. The Lancet, 371, 760-770.
https://doi.org/10.1016/S0140-6736(08)60346-3 - 12. Bump, R.C., Mattiasson, A., Bo, K., Brubaker, L.P., DeLancey, J.O., Klarskov, P., et al. (1996) The Standardization of Terminology of Female Pelvic Organ Prolapse and Pelvic Floor Dysfunction. American Journal of Obstetrics & Gynecology, 175, 10-17.
https://doi.org/10.1016/S0002-9378(96)70243-0 - 13. Bansal, R., Agarwal, M.M. and Modi, M. (2011) Urodynamic Profile of Diabetic Patients with Lower Urinary Tract Symptoms: Association of Diabetic Cystopathy with Autonomi and Peripheral Neuropathy. Urology, 77, 699-705.
https://doi.org/10.1016/j.urology.2010.04.062 - 14. Pfisterer, M.H., Griffiths, D.J. and Schaefer, W. (2006) The Effect of Age on Lower Urinary Tract Function: A Study in Women. Journal of the American Geriatrics Society, 54, 405-412.
https://doi.org/10.1111/j.1532-5415.2005.00613.x - 15. Zimmern, P., Litman, H.J. and Nager, C.W. (2014) Effect of Aging on Storage and Voiding Function in Women with Stress Predominant Urinary Incontinence. The Journal of Urology, 192, 464-468.
https://doi.org/10.1016/j.juro.2014.01.092 - 16. Shin, Y.S., On, J.W. and Kim, M.K. (2015) Effect of Aging on Urodynamic Parameters in Women with Stress Urinary Incontinence. Korean Journal of Urology, 56, 393-397.
https://doi.org/10.4111/kju.2015.56.5.393 - 17. Yang, J.M. and Huang, W.C. (2003) Factors Associated with Voiding Function in Women with Lower Urinary Tract Symptoms: A Mathematic Model Explanation. Neurourology and Urodynamics, 22, 574-581.
https://doi.org/10.1002/nau.10086 - 18. Gilmour, R.F., Churchill, B.M. and Steckler, R.E. (1993) A New Technique for Dynamic Analysis of Bladder Compliance. The Journal of Urology, 150, 1200-1203.
- 19. Toppercer, A. and Tetreault, J.P. (1979) Compliance of the Bladder: An Attempt to Establish Normal Values. Urology, 14, 205.
https://doi.org/10.1016/0090-4295(79)90164-X - 20. Abrams, P. (1999) Bladder Outlet Obstruction Index, Bladder Contractility Index and Bladder Voiding Efficiency: Three Simple Indices to Define Bladder Voiding Function. BJU International, 84, 14-15.
https://doi.org/10.1046/j.1464-410x.1999.00121.x - 21. Wahl, E.F., Lahder-Vasama, T.T. and Churchill, B.M. (2003) Estimation of Glomerular Filtration Rate and Bladder Capacity: The Effect of Maturation, Ageing, Gender and Size. BJU International, 91, 255-262.
https://doi.org/10.1046/j.1464-410X.2003.04053.x - 22. Pfisterer, M.H., Griffiths, D.J., Rosenberg, L., Schaefer, W. and Resnick, N.M. (2007) Parameters of Bladder Function in Pre- , Peri- , and Post-Menopausal Continent Woman without Detrusor Overactivity. Neurourology and Urodynamics, 26, 356-361.
https://doi.org/10.1002/nau.20381 - 23. Pfisterer, M.H., Griffiths, D.J., Rosenberg, L., Schaefer, W. and Resnick, N.M. (2006) The Impact of Detrusor Overactivity on Bladder Function in Younger and Older Women. Journal of Urology, 175, 177-183.
https://doi.org/10.1016/s0022-5347(05)00985-7 - 24. Schultens, A., Becker, T., Balmer, D., Seidilová-Wuttke, D. and Wuttke, W. (2004) In Vivo Properties of the Urinary Bladder Wall and Their Modulation by Estradiol and Raloxifene in a Rat Model. Experimental and Clinical Endocrinology & Diabetes, 112, 514-519.
https://doi.org/10.1055/s-2004-821314 - 25. Lowalekar, S.K., Cristofaro, V., Radisavljevic, Z.M., Yalla, S.V. and Sulivan, M.P. (2012) Loss of Bladder Smooth Muscle Caveolae in the Aging Bladder. Neurourology and Urodynamics, 31, 586-592.
https://doi.org/10.1002/nau.21217 - 26. Schueth, A., Spronck, B., van Zandvoort, M.A. and van Koeveringe, G.A. (2016) Age-Related Changes in Murine Bladder Structure and Sensory Innervation: A Multiphoton Microscopy Quantitative Analysis. Age, 38, 17.
https://doi.org/10.1007/s11357-016-9878-1 - 27. Tseng, L.H., Liang, C.C., Tsay, P.K., Wang, A.C., Lo, T.S. and Lin, Y.H. (2008) Factors Affecting Voiding Function in Urogynecology Patients. Taiwanese Journal of Obstetrics and Gynecology, 47, 417-421.
https://doi.org/10.1016/S1028-4559(09)60009-7



