Soft Nanoscience Letters
Vol.3 No.2(2013), Article ID:29657,7 pages DOI:10.4236/snl.2013.32007
Preparation of PVDF/SiO2 Composite Nanofiber Membrane Using Electrospinning for Polymer Electrolyte Analysis
![]()
Department of Industrial Chemistry, School of Chemical Sciences, Alagappa University, Karaikudi, India.
Email: *pms11@rediffmail.com
Copyright © 2013 M. Sethupathy et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received November 18th, 2012; revised December 19th, 2012; accepted December 28th, 2012
Keywords: Electrospinning Poly(vinylidene fluoride); Silica Nanoparticle; Nanofiber Membrane; Superhydrophobicity
ABSTRACT
Superhydrophobic poly(vinylidene fluoride) PVDF-SiO2 composite membranes with different % of SiO2 contents were prepared by electrospinning. The surface morphologies of the membranes are characterized by using scanning electron microscopy. The nanofibers in the membranes were stacked in layers to produce fully interconnected pores that resulted in high porosity. The incorporation of SiO2 into the nanofiber membrane improved the ionic conductivity from 0.2428 × 10−4 Scm−1 to 7.731 × 10−4 Scm−1 at room temperature. The surface roughness of the membranes increased with increasing the SiO2 content, while the average diameter of nanofibers was rarely affected. Superhydrophobic PVDF membrane with a contact angle larger than 136˚ was prepared by the electrospinning of the SiO2 functionalized PVDF. The surface composition of the membranes is analyzed by using FTIR and the contact angles and water drops on the surface of the membrane are measured. The contact angle experimental results of PVDF-SiO2 composite membranes showed an improvement of hydrophobicity with % of nano SiO2.
1. Introduction
Polymer electrolytes have attracted great interest compared to traditional liquid electrolytes, which provide the advantages to develop lighter and safer batteries with long shelf life, leak proof construction and easy fabrication into desired shape and size [1]. There have been many efforts to develop polymer electrolytes with good ambient temperature conductivity and stable electrode/ electrolyte interfacial properties with minimum resistance to ion transportation [2,3]. The properties of porous host polymer membrane such as pore size, porosity and pore size distribution are strongly dependent on its processing methods. Different methods such as solvent casting, plasticizer extraction and phase inversion have been adopted for the preparation of porous polymer membranes [4-6]. However, polymer membranes made by these methods show low-rate capabilities at high discharge rates because of low-order pore size, low porosity and poor channel in ionic conduction.
Electrospinning is an emerging technique to prepare polymer membranes that are composed of ultrafine fibers with micron and sub-micron diameter. Electrospinning is a process for producing superfine fibrous and porous membranes by forcing a polymer melt or solution through a spinneret with an electric field. The average diameter of the fibers produced by electrospinning is at least 10 - 100 times smaller than the conventional fibers produced by melt spinning [7]. In the electrospinning process, a high electric power is in the range of kilovolts applied to overcome the surface tension of the polymer drops. The applied voltage pulls charges from inside onto the surface of the drop. The molecules in the surface of the drops repel each other and facilitate fresh molecules to reach onto the surface of the drops. Under the right choice of conditions, the drop can become a rapidly growing polymer jet that can follow a tightly looping spiral path. The jet’s width shrinks to as little as few nanometers and its length increases to few micrometers during the process [8].
Electrospun polymer membranes from polymers such as poly(vinylidene fluoride) (PVDF), poly(vinylidene fluoride-cohexafluoropropylene) (PVDF-HFP) and polyacrylonitrile (PAN) are particularly suitable as host matrices for polymer electrolytes [9-11]. The interlaying of thin fibers generates high porosity of over 80% with fully interconnected pore structure and very large surface area-to-volume ratio facilitating high electrolyte uptake and easy transport of ions. Moreover, the resulting polymer electrolyte showed lower bulk impedance and higher rate capability [10].
Inorganic nanoparticles like ZrO2, Al2O3, TiO2 and SiO2 have been used for preparing composite polymer electrolytes [10-12]. These inorganic nanoparticles improve the ionic conductivity of polymer electrolytes by reducing the crystallinity of the host polymer and introduce Lewis acid-base interaction between the polar groups of the inorganic nanoparticles and the electrolyte ionic species [13]. Moreover, inorganic nanoparticles enhance the mechanical properties of polymer electrolytes and the interfacial stability between polymer electrolyte and lithium electrode [14]. Among them, SiO2 has been known as an attractive material for preparing composite polymer electrolytes because it supports the ionic mobility by its starburst shape which effectively influences the order packing tendency of the host polymer chains [15].
PVDF has become a favorable polymer matrix for porous polymer electrolytes due to its appealing properties such as high dielectric constant and strong electronwithdrawing functional groups (-C-F) [16,17]. In the PVDF based porous polymer electrolytes, the absorbed liquid electrolyte is responsible for the ionic conduction while the PVDF matrix acts as the supporting backbone that separates the electrodes. However, mechanical strength is often sacrificed to obtain sufficient conductivity for commercial usage when the amount of liquid electrolyte is increased [18]. Nevertheless, the leakage of electrolyte solution remains due to a phase separation between the polymer matrix and the absorbed electrolyte solution. The loss of electrolyte solution may also lead to a failing of the electrode/electrolyte contact as well as a reduction of ionic conductivity [19]. Therefore, it is of great importance to search for novel safe polymer electrolytes to create a new generation of high performance lithium batteries.
In recent years, electrospinning technology is used for the preparation of highly porous membranes that ideally suits for the application as polymer electrlytes/separators in lithium batteries [20,21]. Several electrospun membranes based on P(VdF-HFP) were reported [22-25]. These membranes showed ionic conductivity in the order of mScm−1 at room temperature and were electrochemically stable at potentials higher than 4.5 V versus Li/Li+. Recently, nanosize SiO2 particles were incorporated into P(VdF-HFP) membrane during electrospinning [26]. An ionic conductivity of 7.731 × 10−4 Scm−1 was achieved. In the present study, an attempt was made to develop a new type of organic-inorganic composite nanofiber membranes based on PVDF-SiO2 via electrospinning method. The SiO2-dispersed PVDF nanofiber membranes led to excellent morphology suitable for the enhancement of electrochemical performance by providing large surface area, good electrolyte uptake, high contact angle and high ionic conductivity.
2. Experimental Deatails
2.1. Preparation of Electrospun Nanofiber Membranes
Poly(vinylidene fluoride) (PVDF), fumed silica (SiO2), N,N-dimethylformamide (DMF), lithium hexafluorophosphate (LiPF6), ethylene carbonate (EC) and dimethyl carbonate (DMC) were purchased from Sigma-Aldrich Co. and used without further purification. For SiO2, the particle size is 70 nm and the surface area is about 390 m2·g−1. Other reagents and solvents were commercially purchased and used as received.
The electrospinning setup utilized in this study consisted of a syringe and needle (ID = 0.4 mm), a ground electrode and a high voltage supply (ZEONICS SYSTECH HIGH VOLTAGE D.C. P/S). The needle was connected to the high voltage supply, which could generate positive DC voltages up to 40 kV. For the electrospinning of PVDF-SiO2 composite nanofiber membranes, PVDF was first dissolved in DMF at a concentration of 18 w/v% and then SiO2 was mixed with different concentration under 60˚C for 12 hours. The contents of SiO2 in the composite solutions were 0, 0.3%, 0.5%, and 0.7 wt% based on the weight of PVDF. The composite solution held in a 5 ml syringe was delivered into a needle spinneret by a syringe pump (KDS 100, KD Scientific Inc.) with a mass flow rate of 1.5 ml·h−1. The steel needle was connected to an electrode of a high voltage supply and a grounded Aluminium foil was placed at 15 cm distance from the needle tip to collect the nanofiber membranes. The positive voltage applied to the composite solutions was 18 kV. All the experiments were carried out at room temperature and below 60% RH. After the electrospinning, the nanofiber membranes were carefully peeled off from the Aluminium foil and put into oven under 40˚C for 12 hours.
2.2. Characterization Techniques
The morphology of the electrospun PVDF-SiO2 membrane was observed using scanning electron microscopy (studied by computer controlled Hitachi S3000 H SEM) under vaccum condition. The sample was sputtered with thin film of gold prior to the SEM measurement.
FTIR spectra of the samples were obtained with a PE IR SPECTRUM ASCII PEDS 1.60 in the wavenumber range 400 - 4000 cm−1. The electrospun membrane was soaked in 1 M LiPF6 in EC/DMC (1:1, v/v). An ionic conductivity cell was assembled by sandwiching a given polymer electrolyte between two stainless steel (SS) blocking electrode (1 × 1 cm). The ionic conductivities were measured by the impedance method of the SS/ polymer electrolyte/SS at room temperature, using Ecochemie-Potentiostat/Galvanostat for impedance studies (Model: Autolab PGSTAT 30, The Netherlands) over the frequency range of 100 mHz to 2 MHz. The electrolyte resistance R was measured using a FRA (Autolab PGSTAT 30, Netherlands) with a 1260 frequency response analyzer controlled by a computer. The ac oscillation was 10 mV. The data were analyzed by Z-plot software. The ionic conductivity of the membrane σ was then calculated by the equation σ = l/RA, where l is the thickness of the film, R is the resistance electrolyte and A is the area of the film. The wettability of thin film of PVDF-SiO2 composites (0.3%, 0.5%, and 0.7%) were determined by contact angle measurement using VCA optima, ACT product, INC. Hydrophilic or Hydrophobic characteristic nature of samples were estimated by contact angle (q) value. The wetting liquid was Millipore-grade distilled water (liquid surface tension (gl) = 72.8 mJ·m−2) [27]. 5 ml of water was added uniformly over the surface of the membrane. Contact angle was measured after 1 minute of water addition to provide the configuration between the measurements. When a drop of liquid is brought into contact with a flat solid surface, the final shape taken by the drop is expressed by “q”. The increased value of cosq denoted higher wettability characteristics of a material.
3. Results and Discussion
3.1. PVDF-SiO2 Membrane Preparation and Their Morphological Studies
The electrospinning was performed with 18 w/v% poly(vinylidene fluoride) (PVDF) solution in order to obtain nanofiber membranes that consist of bead-free fibers with uniform size and well-defined morphology. In the electrospinning of the polymer solution, the molecular chain entanglements prevent the breakup of the electrically driven jet into individual droplets, so that electrostatic stresses allow the jet to elongate and be deposited as ultrafine nanofiber membranes on the collector. PVDFSiO2 composite nanofiber membranes were prepared by electrospinning from PVDF-SiO2 solutions with different % of SiO2 contents. The % of SiO2 in the mixed solutions were 0 (PVDF), 0.3 wt% (SiO2—0.3 wt%), 0.5 wt% (SiO2—0.5 wt%) and 0.7 wt% (SiO2—0.7 wt%) based on the weight of PVDF used.
The surface morphology of electrospun composite nanofiber membranes are shown in Figure 1. The nanofiber membranes exhibited a fully interconnected membrane with nanosize distribution. The surface roughness increased with increasing the SiO2 content, while the average diameter of nanofibers was rarely affected. Due to confirming strong peak from FTIR and surface roughness through the SEM that SiO2 presents in the polymer matrix.
3.2. FTIR Studies
FTIR analysis was carried out in the range of 400 - 4000 cm−1 to understand and confirm the presence of functional groups in the PVDF-SiO2 nanofiber membranes. As shown in Figure 2 three strong peaks are observed at the range of 1392, 854 and 1178 cm−1. The former two peaks were assigned due to the C-F stretching vibration and the latter one peak was assigned due to the C-C bond of the PVDF. In the case of the composite nanofiber membrane, the peak observed at 478 cm−1 may be associated with Si-O-Si stretching vibration of SiO2.
3.3. Conductivity Studies
The ionic conductivities of the nanofiber membranes were calculated at room temperature by the impedance method. Figure 3 shows the impedance data of polymer electrolytes based on electrospun nanofiber membranes with or without SiO2. The impedance responses are typical of electrolytes with a major part towards total resistance from bulk resistance and only a minor contribution from grain boundary resistance. The real-axis representing the electrolyte/electrode double layer capacitance was obtained for all samples over the whole range of frequency evaluated. The intercept on the real-axis exhibiting bulk resistance varies between 0.242  and 7.731
and 7.731 , which reduces with increasing the SiO2 content in the nanofiber membranes.
, which reduces with increasing the SiO2 content in the nanofiber membranes.
From the impedance data, the ionic conductivities of the nanofiber membranes at room temperature were calculated and presented in Figure 4. The ionic conductivity largely depends on the pore structure that entraps liquid electrolytes and hence the formation of pores in membranes is very important for obtaining proper channel of ionic conduction [28]. The ionic conductivities of all samples were higher than 0.242 × 10−4 Scm−1 because of interwoven structure introduced during electrospinning. In addition, the incorporation of SiO2 into the nanofiber membrane improved the ionic conductivity from 0.242 × 10−4 Scm−1 to 7.731 × 10−4 Scm−1.
3.4. Electrolyte Uptake
At presents a relationship of the electrolyte uptake of the nanofiber membranes. The data was obtained by soaking
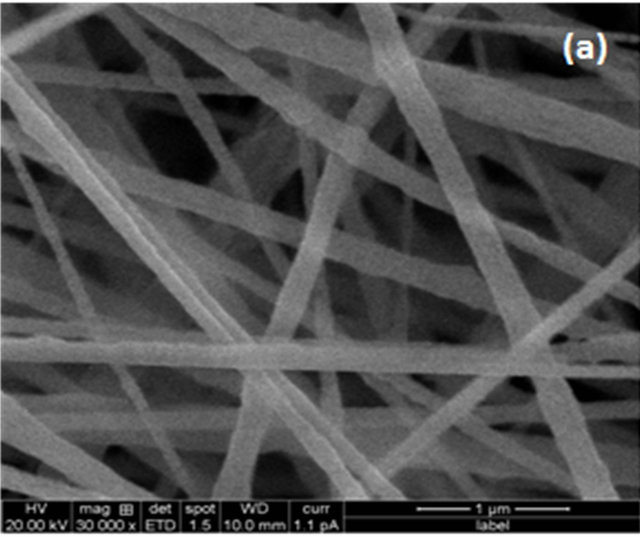
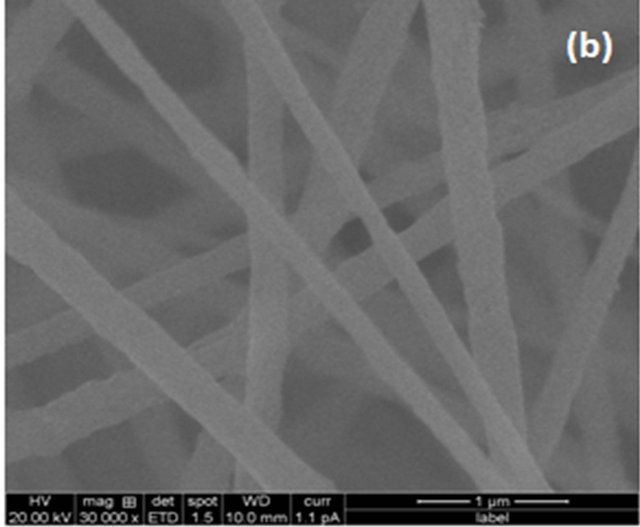


Figure 1. SEM images of electrospun composite nanofiber membranes with different SiO2 contents: (a) Pure PVDF; (b) SiO2 0.3 wt%; (c) SiO2 0.5 wt%; (d) SiO2 0.7 wt%.
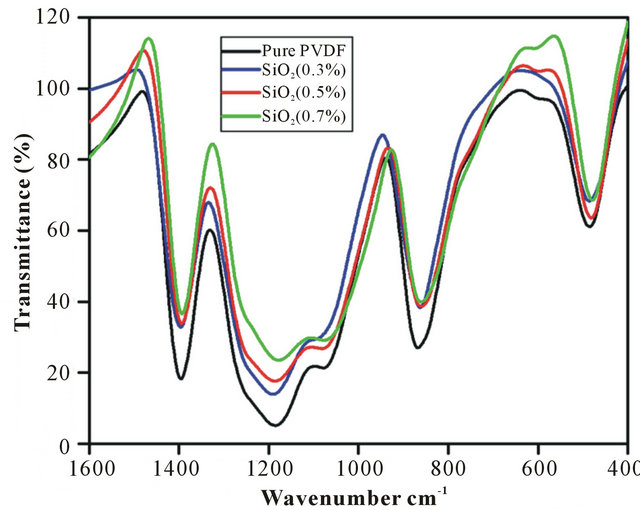
Figure 2. FTIR Spectra of 1) Pure PVDF; 2) SiO2 0.3 wt%, 3) SiO2 0.5 wt%; 4) SiO2 0.7 wt% .
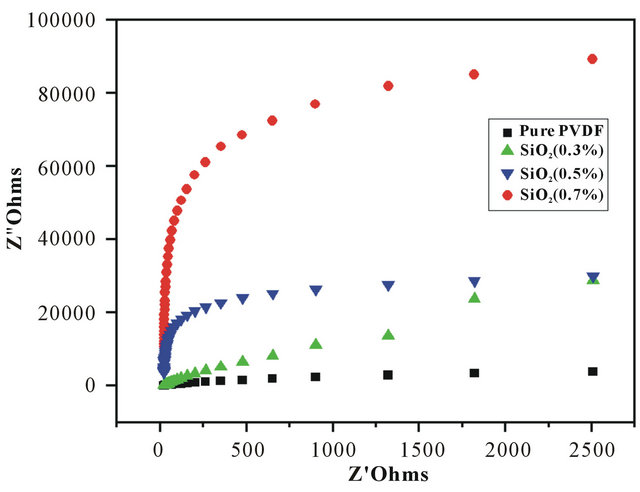
Figure 3. Impedance spectra of polymer electrolyte based on the nanofiber membranes with different SiO2 contents 1) Pure PVDF; 2) SiO2 0.3wt%; 3) SiO2 0.5 wt%; 4) SiO2 0.7 wt% .
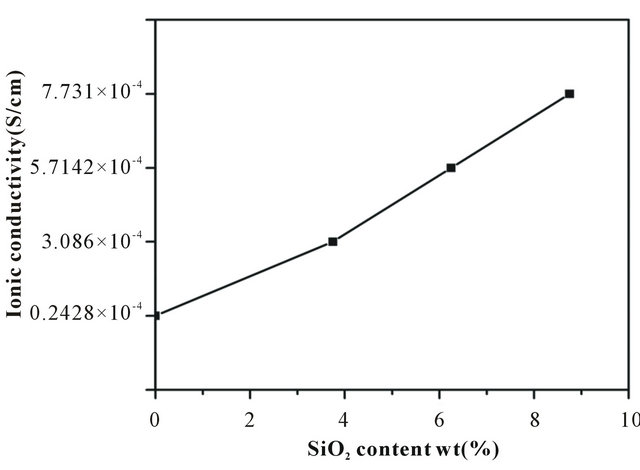
Figure 4. Variations of ionic conductivity in the nanofiber membranes with different SiO2 contents.
the nanofiber membranes in the liquid electrolyte of 1 M LiPF6 for a period of 1 h. The electrolyte uptake is observed to increase steadily with the SiO2. In the case of SiO2 0.7 wt% membrane, the electrolyte uptake reached to ~450%.
The high retention ability and faster penetration of liquid electrolyte into the fibrous membranes are due to the unique pores generated from the interconnected fibers. So that pores and voids are increases the ionic conductivity also increases. Hence the uptake process is stabilized within the initial 10 min. PVDF-SiO2 membrane (0.7 wt%) showed the highest value ~450%., because of its uniform fiber diameter and high specific surface area. As shown in Figure 5.
3.5. Wettability Test
Contact angles are commonly specified in degrees, the test fluid used in the measurements must be stated, for its surface tension will affect the angle (Young’s equation). Contact angles are used to predict wettability and adhesion, and to indicate monolayer overage of adsorbed or deposited films. Another use of contact angles is to estimate “surface free energy” by using such theories as the Girifalco, Owens-Wendt, Wu, or Lewis Acid/Base models.
Although contact angles of upto 127.10˚, 133.33˚, 134.80˚and 136.50˚ can be reached by directly electrospinning PVDF and SiO2 composite solutions, respectively. Therefore, PVDF and SiO2 composite were used to successfully prepare superhydrophobic membranes with high contact angles of 134.80˚and 136.50˚ respectively. Membranes prepared by electrospinning method have much higher contact angle than because of the high surface area of the formed fibers that ranges from nanometer to submicron scale. As shown in Figure 6.
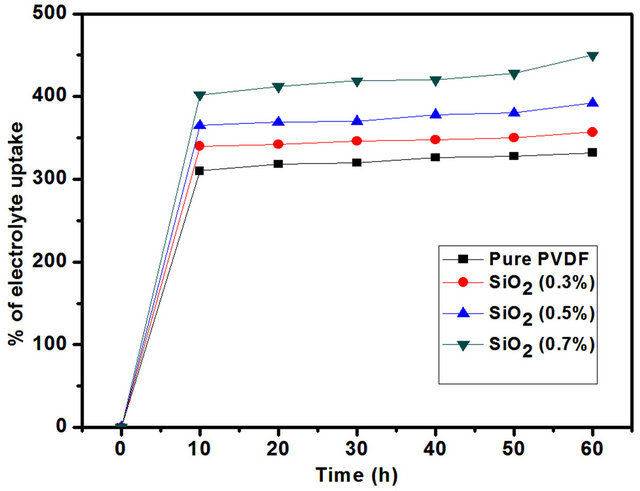
Figure 5. Electrolyte uptake of the nanofiber membrane with different SiO2 contens.
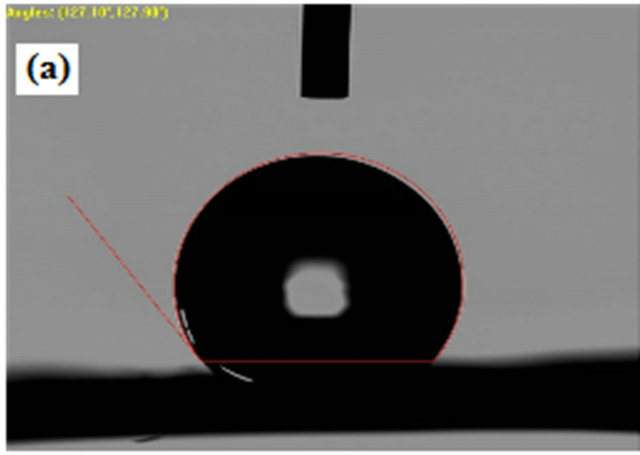
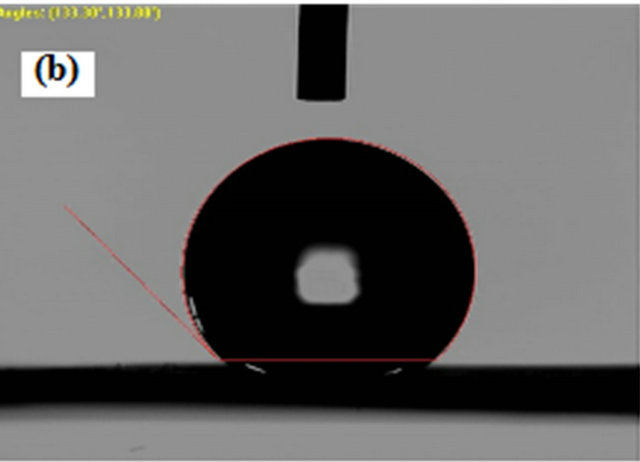
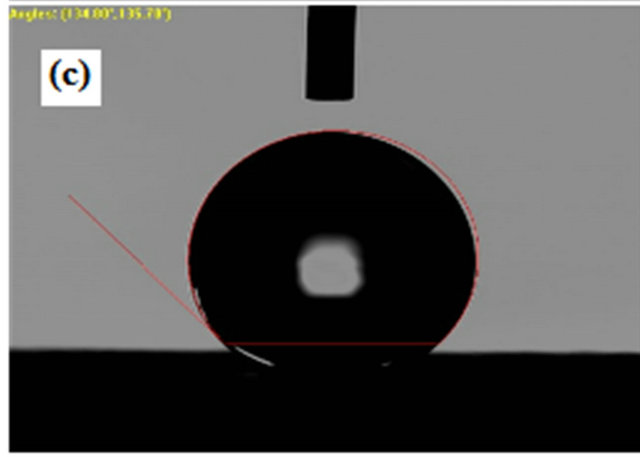

Figure 6. Contact angle measurement on (a) Pure PVDF; (b) SiO2 0.3wt%; (c) SiO2 0.5 wt%; (d) SiO2 0.7 wt%.
4. Conclusion
In the present study, organic-inorganic composite nanofiber membranes were prepared by electrospinning of PVDF solutions with different nano SiO2 content, which showed a porous morphology formed by interlaying of the fibers. The presence of the SiO2 nanoparticles improved the conductivity and wettability of the nanofiber membranes. The introduction of nano SiO2 created amorphous regions by the way of the interactions between the SiO2 surfaces and the polymer chains. The ionic conductivity of the membranes increased with increasing the SiO2 content at different concentration. The electrolyte uptake reached to ~450%. The high retention ability and faster penetration of liquid electrolyte into the fibrous membranes are due to the unique pores generated from the interconnected fibers. It was observed that both PVDF and SiO2 composite membranes can only reach contact angles lower than 127.10˚ by either coupling or by electrospinning pure PVDF and SiO2 composite solutions. A superhydrophobic membrane with contact angles of 136.50˚ was achieved by electrospun PVDF-SiO2 composite nanofiber membrane. It is concluded that the composite nanofiber membranes fabricated by electrospinning offer a new approach for application in highperformance lithium batteries. Superhydrophobic PVDF membranes were prepared by electrospinning of fumed silica functionalized PVDF.
5. Acknowledgements
Author would like to thank to AURF Project, Alagappa University Karaikudi, Tamil Nadu, India. UGC-New Delhi, India for providing UGC-BSR-RFMS Fellowship to the Department of Industrial Chemistry to carry out this work.
REFERENCES
- J. Y. Xi, X. P. Qiu, X. Z. Tang, W. T. Zhu and L. Q. Chen, “PVDF-PEO Blends Based Microporous Polymer Electrolyte: Effect of PEO on Pore Configurations and Ionic Conductivity,” Journal of Power Sources, Vol. 157, No. 1, 2006, pp. 501-506. doi:10.1016/2005.08.009
- Z. Gadjourova, Y. G. Andreev, D. P. Tunstall and P. G. Bruce, “Ionic Conductivity in Crystalline Polymer Electrolytes,” Nature, Vol. 412, 2001, p. 520. doi:10.1038/35087538
- C. Wang, T. Sakai, O. Watanabe, K. Hirahara and T. Nakanishi, “All Solid-State Lithium-Polymer Battery Using a Self-cross-Linking Polymer Electrolyte,” Journal of Electrochemical Society, Vol. 150, No. 9, 2003, pp. 1166-1170. doi:10.1149/1.1593652
- Z. Wang and Z .Tang, “Characterization of the Polymer Electrolyte Based on the Blend of Poly(vinylidene fluoride-co-hexafluoropropylene) and Poly(vinyl pyrrolidone) for Lithium Ion Battery,” Materials Chemistry and Physics, Vol. 82, No. 1, 2003 pp. 16-20.
- J. W. Choi, J. H. Kim, G. Cheruvally, J. H. Ahn, K. W. Kim, H. J. Ahn and J. U. Kim, “Microporous Poly(vinylidene fluoride-co-hexafluoropropylene) Polymer Electrolytes for Lithium/Sulfur Cells,” Journal of Industrial and Engineering Chemistry, Vol. 12, No. 6, 2006, pp. 939-949.
- W. H. Ru, X. M. He, L. Wang, C. Y. Jiang and C. R. Wan, “Preparation of PVDF-HFP Microporous Membrane for Li-Ion Batteries by Phase Inversion,” Journal of Membrane Science, Vol. 272, No. 1-2, 2006, pp. 11-14.
- G. Srinivasan and D. H. Reneker, “Structure and Morphology of Small Diameter Electrospun Aramid Fibres,” Polymer International, Vol. 36, 1995, pp. 195-201. doi:10.1002/pi.1995.210360210
- D. Adam, “A Fine Set of Threads,” Nature, Vol. 411, 2001, p. 236. doi:10.1038/35077170
- S. W. Lee, S. W. Choi, S. M. Jo, B. D. Chin, D. Y. Kim and K. Y. Lee, “Electrochemical Properties and Cycle Performance of Electrospun Poly(vinylidene fluoride)- Based Fibrous Membrane Electrolytes for Li-Ion Polymer Battery,” Journal of Power Sources, Vol. 163, No. 1, 2006, pp. 41-46. doi./10.1016/2005.11.102
- P. Raghvan, J. W. Choi, J. H. Ahn, G. Cheruvally, G. S. Chauhan, H. J. Ahn and C. W. Nah, “Novel Electrospu Poly(vinylidene fluoride-co-hexafluoropropylene)-in Situ SiO2 Composite Membrane-Based Polymer Electrolyte for Lithium Batteries,” Journal of Power Sources, Vol. 184, No. 2, 2008, pp. 437-443.
- H. R. Jung, D. H. Ju, W. J. Lee, X. Zhang and R. Kotek, “Electrospun Hydrophilic Fumed Silica/Polyacrylonitrile Nanofiber-Based Composite Electrolyte Membranes,” Electrochim Acta, Vol. 54, No. 13, 2009, pp. 3630-3637. doi:10.1016/j2009.01.039
- P. Raghavan, X. Zhao, J. K. Kim, J. Manuel, G. S. Chauhan, J. H. Ahn and C. W. Nah, “Ionic Conductivity and Electrochemical Properties of Nanocomposite Polymer Electrolytes Based on Electrospun Poly(vinylidene fluoride-co-hexafluoropropylene) with Nano-Sized Ceramic Fillers,” Electrochim Acta, Vol. 54, No. 2, 2008, pp. 228-234.
- S. H. Chung, Y. Wang, L. Persi, F. S. Croce, G. Greenbaum, B. Scrosati and E. Plichta, “Enhancement of Ion Transport in Polymer Electrolytes by Addition of Nanoscale Inorganic Oxides,” Journal of Power Sources, Vol. 97-98, 2001, pp. 644-648. doi./10.1016/S0378-7753(01)00748-0
- G. Jiang, S. Maeda, H. Yang, Y. Saito, S. Tanase and T. Sakai, “All Solid-State Lithium-Polymer Battery Using Poly(urethane acrylate)/Nano-SiO2 Composite Electrolytes,” Journal of Power Sources, Vol. 141, No. 1, 2005, pp.143-148.
- Y. Liu, J. Y. Lee and L. Hong, “Functionalized SiO2 in Poly(ethylene oxide)-Based Polymer Electrolytes,” Journal of Power Sources, Vol. 109, No. 2, 2002, pp. 507-514. doi:10.1016/S0378-7753(02)00167-2
- C. Y. Chiang, Y. J. Shen, M. J. Reddy and P. P. Chu, “Complexation of Poly(vinylidene fluoride): LiPF6 Solid Polymer Electrolyte with Enhanced Ion Conduction in ‘Wet’ Form,” Journal of Power Sources, Vol. 123, No. 2, 2003, pp. 222-229. doi:10.1016/S0378-7753(03)00514-7
- N. S. Mohamed and A. K. Arof, “Investigation of Electrical and Electrochemical Properties of PVDF-Based Polymer Electrolytes,” Journal of Power Sources, Vol. 132, No. 1-2, 2004, pp. 229-234. doi:10.1016/.2003.12.031
- M. Oliver, “Blended Polymer Gel Electrolytes,” US Patent No. 5658685, 1997.
- Y. J. Kim, C. H. Ahn, M. B. Lee and M. S. Choi, “Characteristics of Electrospun PVDF/SiO2 Composite Nanofiber Membranes as Polymer Electrolyte,” Journal of Materials Chemistry and Physics, Vol. 127, No. 1-2, 2011, pp. 137-142. doi:10.1016/S0254-0584(01)00400
- A. I. Gopalan, K. P. Lee, K. M .Manesh and P. Santhosh, “Poly(vinylidene fluoride)-Polydiphenylamine Composite Electrospun Membrane as High Performance Polymer Electrolyte for Lithium Batteries,” Journal of Membrane Science, Vol. 318, No. 1-2, 2008, pp. 422-428. doi:10.1016/2008.03.007
- K. P. Lee, A. I. Gopalan, K. M. Manesh, P. Santhosh and K. S. Kim, “Influence of Finely Dispersed Carbon Nanotubes on the Performance Characteristics of Polymer Electrolytes for Lithium Batteries,” IEEE Transactions on Nanotechnology, Vol. 6, No. 3, 2007, pp. 362-367.
- G. Cheruvally, J. K. Choi J. W. Kim, J. H. Ahn, Y. J. Shin, J. Manuel, P. Raghavan, K. W. Kim, H. J. Ahn, D. S. Choi and C. E. Song, “Electrospun Polymer Membrane Activated with Room Temperature Ionic Liquid: Novel Polymer Electrolytes for Lithium Batteries,” Journal of Power Sources, Vol. 172, No. 2, 2007, pp. 863-869. doi:10.1016/2007.07.057
- S. W. Choi, J. R. Kim, Y. R. Ahn, S. M. Jo and E. J. Cairns, “Characterization of Electrospun PVDF FiberBased Polymer Electrolytes,” Chemistry of Materials, Vol. 19, No. 1, 2007, pp. 104-115. doi:10.1021/cm060223+
- X. Li, G. Cheruvally, J. K. Kim, J. W. Choi, J. H. Ahn, K. W. Kim and H. J. Ahn, “Polymer Electrolytes Based on an Electrospun Poly(vinylidene fluoride-co-hexafluoro propylene) Membrane for Lithium Batteries,” Journal of Power Sources, Vol. 167, No. 2, 2007, pp. 491-498.
- K. Gao, X. Hu, C. Dai and T. Yi, “Crystal Structures of Electrospun PVDF Membranes and Its Separator Application for Rechargeable Lithium Metal Cells,” Materials Science Engineering B, Vol. 131, No. 1-3, 2006, pp. 100- 105. doi:10.1016/2006.03.035
- J. K. Kim, G. Cheruvally, X. Li, J. H. Ahn, K. W. Kim and H. J. Ahn, “Preparation and Electrochemical Characterization of Electrospun, Microporous Membrane-Based Composite Polymer Electrolytes for Lithium Batteries,” Journal of Power Sources, Vol. 178, No. 2, 2008, pp. 815-820. doi:10.1016/2007.08.063
- E. Lugscheider and K. Bobzin, “Wettability of PVD Compound Materials by Lubricants,” Surface & Coating Technology, Vol. 165, No. 1, 2003, pp. 51-57. doi:10.1016/S0257-8972(02)00724-7
- S. Rajendran, O. Mahendran and R. Kannan, “Lithium Ion Conduction in Plasticized PMMA-PVDF Polymer Blend Electrolytes,” Materials Chemistry & Physics, Vol. 74, No. 1, 2002, pp. 52-57. doi:10.1016/S0254-0584(01)00400
NOTES
*Corresponding author.

