International Journal of Organic Chemistry
Vol. 2 No. 1 (2012) , Article ID: 17842 , 8 pages DOI:10.4236/ijoc.2012.21002
An Efficient Synthesis of Pyrido[2,3-d]pyrimidine Derivatives via One-Pot Three-Component Reaction in Aqueous Media
1Department of Chemistry, Islamic Azad University, East Tehran Branch (Qiam Dasht), Tehran, Iran
2Peptide Chemistry Research Center, K. N. Toosi University of Technology, Tehran, Iran
Email: *balalaie@yahoo.com
Received December 6, 2011; revised January 6, 2012; accepted January 17, 2012
Keywords: Diammonium Hydrogen Phosphate (DAHP); Microwave Irradiation (MWI); Water in Organic Synthesis; Pyrido[2,3-d]pyrimidine
ABSTRACT
A series of pyrido[2,3-d]pyrimidines derivatives have been prepared by one-pot three-component reaction of 4(6)- aminouracil, malononitrile and aromatic aldehydes. This efficient synthesis was done under microwave irradiation conditions (method A) and also using catalytic amount of diammonium hydrogen phosphate [(NH4)2HPO4] (DAHP) in aqueous media (method B). This procedure has the advantages of good yields, easy work-up, and benign environmentally friendly character. Reaction could proceed via domino Knoevenagel-Michael-cyclization reactions.
1. Introduction
Pyridopyrimidine and its derivatives have been studied due to a variety of chemical and biological significance. The importance of pyridopyrimidines as biologically active compounds includes their use as antibacterial [1-3], antiallergic [4], antitumor [2,3] antifolate [5], tyrosine kinase [6], antimicroibial [7], calcium channel antagonists [8], antibacterial [9-12], anti-inflammatory, analgesic [13], antihypertensive [14], antileishmanial [15], tuber-culostatic [16], anticonvulsants [17], diuretic, potassiumsparing [18], and antiaggressive activities [19]. The need to reduce the amount of toxic waste and byproduct arising from chemical processes requires increasing emphasis on the use of less toxic and environmentally compatible materials in the design of new synthetic methods. One of the most promising approaches is using water as the reaction media [20-26].
Several approaches have been developed for the synthesis of pyridopyrimidines such as: 1) the reaction of benzylidene derivatives of malononitrile with 6-amino-3, 4-dihydropyrimidine in refluxing ethanol [27,28]; 2) the reaction of 6-amino-1-thio uracil with ethyl-3-phenyl-2- cyanoacrylate in absolute ethanol and in the presence of Et3N by heating [29,30]; 3) the three-component reaction of aldehydes, alkyl nitriles and aminopyrimidines in water and in the presence of KF-Al2O3 as catalyst [31]; 4)
the similar three-component reaction catalyzed by TEBAC [32] or reaction of amino-uracil with α,β-unsaturated compounds in ionic liquid at 90˚C [33]. Some of the reported methods have their merit such as: 1) multi-step synthesis with the use of expensive harmful reagents and 2) low yields. Thus, the development of efficient method for the synthesis of biologically active compounds such as pyrido[2,3-d]pyrimidines, in one-step would be highly valuable and desirable.
2. Methods
The utility of microwave energy in synthetic organic chemistry has been increasingly recognized in recent years. It was shown that MWI-irradiated multi-component reactions have constituted an especially attractive synthetic strategy for rapid and efficient library generation. It has some advantages such as environmentally friendly, improving the bond forming efficiency (BFE), time saving, experimental simplicity, and also in view of atom economy, multi-component reaction is preferred [34-36] and this approach was used for academic and industries research [37]. Meanwhile, there has been increasing interest in the development of new catalysts, which are cheap, and effective in aqueous media. Recently, diammonium hydrogen phosphate [(NH4)2HPO4] (DAHP) in aqueous media has emerged as a very effective catalyst for various organic transformation, and our group has been developing organic synthesis in aqueous media. DAHP is very inexpensive, water soluble, non-toxic and commercially available so it can be used in the laboratory without special precautions [38]. This encouraged us to consider DAHP as an ideal catalyst for the one-pot synthesis of pyrido[2,3-d]pyrimidines.
Due to the potential interest in finding more new versatile procedures, a microwave-assisted synthesis and using DAHP as a mild catalyst in aqueous media was investigated.
3. Results and Discussion
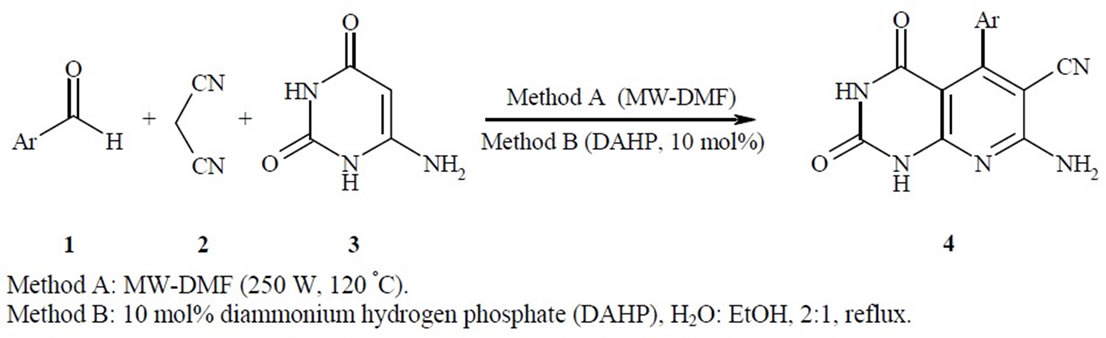
In this context, we introduce an efficient one-pot threecomponent reaction of aromatic aldehydes 1, malononitrile 2, and 4(6)-aminouracil 3 and MWI for the synthesis of pyrido[2,3-d]pyrimidines using microwave irradiation (Scheme 1, method A) and also in the presence of catalytic amounts of DAHP (10 mol%) in refluxing aqueous ethanol (Scheme 1, method B)
With the aim to develop more efficient processes, reduce the number of separate reaction steps, and minimize byproducts for the synthesis of pyrido[2,3-d]pyrimidines [39,40], and in continuation of our previous work on the development of new and efficient methods for the preparation of heterocyclic compounds [41-45], a convenient, practical, inexpensive, rapid procedure for the preparation of pyrido[2,3-d]pyrimidine derivatives 4(a-h) was reported (Scheme 1, methods A, B).
To explore the scope and versatility of this method, various solvents were investigated. We used the reaction of 4-nitrobenzaldehyde (1h, 1 mmol), malononitrile (2, 1.2 mmol) and 4(6)-aminouracil (3, 1 mmol) for the preparation of compound 4h as a model and various solvents such as glacial acetic acid (HOAc), ethanol, glycol, water and N,N-dimethylformamide (DMF) as solvent
(1.0 mL) were used, also reaction was checked in solventfree conditions at 120˚C respectively. All the reactions were carried out at the maximum power of 250 W. The results are summarized in Table 1. The heating characteristics of a solvent under microwave irradiation conditions are dependent on the dielectric properties of the solvent. This fact is shown in Table 1, where the best result was achieved using DMF as solvent. So DMF was chosen as the reaction solvent. More over in order to optimize the other reaction conditions the different powers and temperatures for the same reaction were examined, so microwave irradiation at 250 W gave the highest yield and the maximum temperature reached during the reaction was 120˚C. Therefore, microwave power of 250 W was chosen as the optimum power.
Table 2 shows the results obtained in the reaction of a series of representative aldehydes 1 with malononitrile (2, 1.2 mmol) and 4(6)-aminouracil (3, 1 mmol) under microwave irradiation. In method B we have used aqueous media catalyzed by DAHP at reflux conditions for the preparation of corresponding products 4(a-h). In this manner and in order to optimize the reaction conditions the catalytic amount of DAHP was varied finding that 10 mol% of DAHP afforded the best yields. It is important to note that in the absence of DAHP the reaction times are increased, meanwhile the yields are decreased mainly. The results are summarized in Table 2. In order to optimize the reaction conditions the catalytic amount of DAHP was varied finding that 10 mol% of DAHP afforded the best yields. It is important to note that in the absence of DAHP the reaction did not take place at all. To show that DAHP is an efficient catalyst rather than a mild base, we adjusted the reaction conditions to pH 8, but we found that the reaction did not proceed.
Scheme 1. Synthesis of 7-amino-2,4-dioxo-5-aryl-1,2,3,4-tetrahydropyrido[2,3-d]pyrimidine-6-carbonitrile.
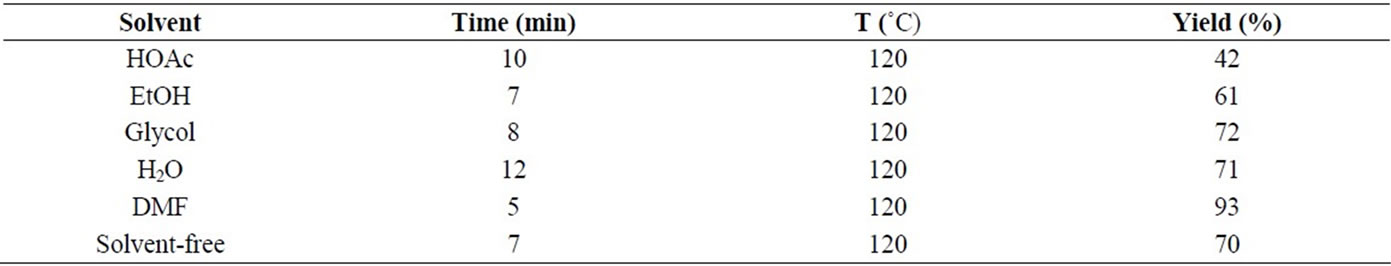
Table 1. Optimization of reaction conditions for the synthesis of compound 4h under microwave irradiation.
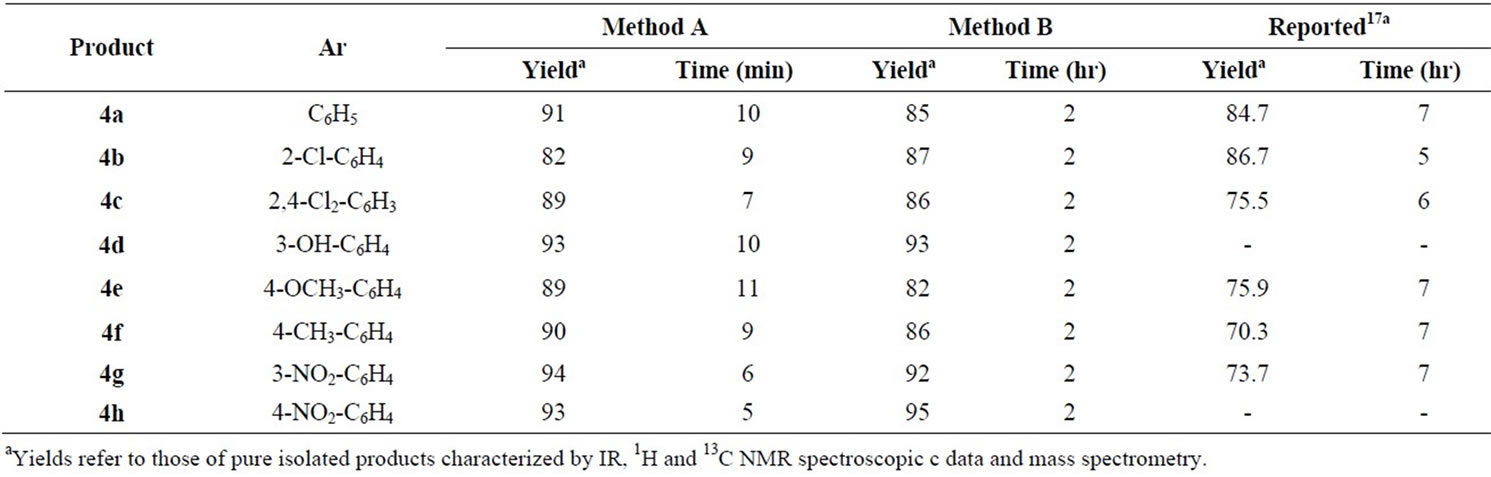
Table 2. Synthesis of 7-amino-2,4-dioxo-5-aryl-1,2,3,4-tetrahydropyrido[2,3-d]pyrimidine-6-carbonitrile 4(a-h) under microwave irradiation (Method A); in aqueous media using DAHP at reflux conditions (Method B).
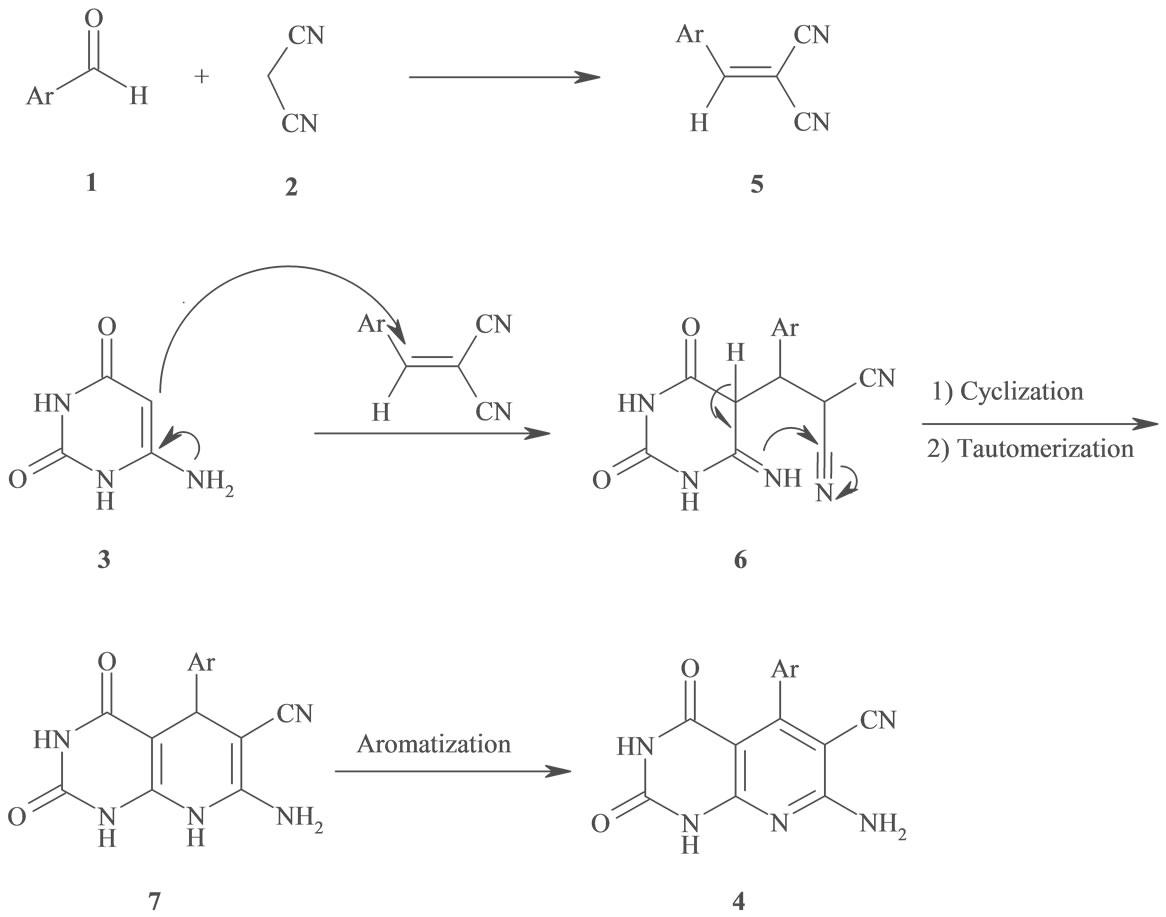
According to a plausible mechanism which is outline in Scheme 2, the formation of 4 is expected to proceed via initial condensation of aldehyde 1 with malononitrile 2 to afford olefin 5, which further undergoes in situ Michael addition with 4(6)-aminouracil 3, to yield intermediate 6, which is then cyclized and subsequently dehydration to afford the aromatized product 4.
Although we have not yet established the mechanism of the one-pot reaction of benzaldehyde derivatives, malononitrile and 4(6)-aminouracil in the presence of DAHP, a possible explanation is given in Scheme 3. We suggest that, DAHP can catalyze the formation of olefin 5 via conversion of aryl aldehyde 1 to a more reactive iminium ion 8, which reacts easily with malononitrile 2 in a Knoevenagel condensation to produce olefin 5 after dehydration of intermediate 9. DAHP can also act as a mild base for the deprotonation of 4(6)-aminouracil 3 to a proposed anion 10, which adds to olefin 5 to generate 4, after proton transfer, tautomerization and aromatization of intermediates 6 and 7 respectively (Scheme 3). This reaction could be categorized as domino Knoevenagel-Michaelcyclization reaction. The structure of compounds 4(a-h) were deduced from their 1H NMR, 13C NMR and IR spectral data and their molecular weight confirmed by mass spectrometry. The mass spectra of these compounds showed the expected molecular ion signals, selected spectroscopic data have been given in general procedure section.
4. Conclusions
In conclusion, we have developed efficient methods for the synthesis of pyrido[2,3-d]pyrimidine derivatives via three-component condensation of aromatic aldehydes, malononitrile and 4(6)-aminouracil in two different reaction conditions; 1) microwave-irradiation; 2) carrying out the reactions in aqueous media and in the presence of catalytic amount of a cheap catalyst (DAHP). The operational simplicity, simple purification procedure, high yields (82% - 95%), environmentally friendly character, and high-speed synthesis (Method A, 5 - 10 min) are advantages of this method compared to previous reported methods.
5. Experimental Section
5.1. General
Melting points were determined with Electrothermal 9100 melting point apparatus and were uncorrected. IR spectra were obtained on an ABB FT-IR (FTLA 2000) spectrometer. 1H NMR and 13C NMR spectra were run on a Bruker DRX-500 AVANCE at 500 and 125 MHz respectively using TMS as internal standard and DMSOd6 as solvent. Mass spectra data were obtained by using GC-MS Hewlet Packard (EI, 70 eV) instrument.
5.2. General Procedure for the Synthesis of 7-Amino-2,4-dioxo-5-aryl-1,2,3,4-tetrahydropyrido[2,3-d]pyrimidine-6-carbonitrile 4(a-h)
Method A. A mixture of aromatic aldehyde 1 (1 mmol), malononitrile (2, 1.2 mmol), 4(6)-aminouracil (3, 1 mmol) and DMF (1.0 mL) were placed into teflon vessel, and subjected to microwave irradiation for a given time at power of 250 W and 120˚C. After completion of the reaction as followed by TLC examination at an interval of 30s, the reaction mixture was cooled to room temperature and then poured in to cold water. The solid product was filtered and washed with boiling water to give the pure product in excellent yield.
Method B. A solution of aromatic aldehyde 1 (1 mmol), malononitrile (2, 1.2 mmol), 4(6)-aminouracil (3, 1 mmol) and diammonium hydrogen phosphate (13.2 mg, 10 mol%) in H2O (10 mL) and Ethanol (5 mL) was stirred at reflux for 2 h. The progress of the reaction was monitored with TLC in 1:1 ethanol-ethyl acetate as TLC solvent. Upon completion of the reaction, the reaction mixture was collected by filtration and purified by wash-
Scheme 2. Proposed mechanism for the one-pot synthesis of 7-amino-2,4-dioxo-5-aryl-1,2,3,4-tetrahydropyrido[2,3-d]pyrimidine-6-carbonitrile under microwave irradiation.
ing with boiling water to afford the corresponding products in high yields.
7-amino-2,4-dioxo-5-phenyl-1,2,3,4-tetrahydropyrido[2,3-d]pyrimidine-6-carbonitrile (4a).
White solid, Mp. > 300˚C, IR (KBr, cm−1): 3403, 3331 (NH2), 3174 (2NH br), 2224 (CN), 1707, 1643 (2CO). 1H NMR (500 MHz, DMSO): δ 7.24 (m, 2H, HAr), 7.40 (m, 3H, HAr), 7.59 (br s, 2H, NH2), 10.89 (s, 1H, NH), 11.44 (s, 1H, NH) ppm, 13C NMR (DMSO) δ: 88.7, 98.3, 115.5, 127.5, 127.7, 128.3, 136.8, 150.3, 155.6, 159.0, 160.1, 160.9; MS: (M+) m/z, 279, 278, 235, 208, 118, 77, 57, 43.
7-amino-2,4-dioxo-5-(2-chlorophenyl)-1,2,3,4-tetrahydropyrido[2,3-d]pyrimidine-6-carbonitrile (4b).
White solid, Mp. > 300˚C (Dec.), IR (KBr, cm−1): 3390, 3311 (NH2), 3188, 3091 (2NH), 2228 (CN), 1699, 1648 (2CO). 1H NMR (500 MHz, DMSO): δ 7.28 (dd, 1H, HAr, J = 7.4, 1.5 Hz), 7.38 (t, 1H, HAr, J = 7.9 Hz), 7.43 (dt, 1H, HAr, J = 7.4, 1.5 Hz), 7.51 (d, 1H, HAr, J = 7.9 Hz,), 7.75 (br s, 2H, NH2), 10.99 (s, 1H, NH), 11.55 (s, 1H, NH) ppm, 13C NMR (DMSO) δ: 88.3, 98.5, 114.8, 126.9, 128.8, 129.0, 129.9, 130.5, 135.9, 150.1, 155.4, 155.8, 159.7, 160.9. MS: (M+) m/z, 313, 278, 188, 153, 111, 77, 57, 43.
7-amino-2,4-dioxo-5-(2,4-dichlorophenyl)-1,2,3,4-tetrahydropyrido[2,3-d]pyrimidine-6-carbonitrile (4c).
White solid, Mp. > 300˚C (Dec.), IR (KBr, cm−1): 3377, 3318 (NH2), 3143, 3068 (2NH), 2206 (CN), 1699, 1648 (2CO). 1H NMR (500 MHz, DMSO): δ 7.34 (d, 1H, HAr, J = 8.2 Hz), 7.50 (d, 1H, HAr, J = 8.2 Hz), 7.72 (br s, 1H, HAr), 7.81 (br s, 2H, NH2), 11.06 (s, 1H, NH), 11.58 (s, 1H, NH) ppm, 13C NMR (DMSO) δ: 88.1, 98.4, 114.7, 127.2, 128.4, 130.3, 131.7, 133.7, 135.0, 150.1, 154.6, 155.4, 159.8, 160.9. MS: (M+) m/z, 348, 312, 277, 77, 57, 43.
7-amino-2,4-dioxo-5-(3-hydroxyphenyl)-1,2,3,4-tetrahydropyrido[2,3-d]pyrimidine-6-carbonitrile (4d).
Brick-red solid, Mp. > 300˚C (Dec.), IR (KBr, cm−1): 3391, 3322 (NH2), 3164, 3071 (2NH), 2237 (CN), 1686, 1649 (2CO). 1H NMR (500 MHz, DMSO): δ 6.59 (s, 1H, HAr), 6.62 (d, 1H, HAr, J = 7.4 Hz), 6.78 (dd, 1H, HAr, J = 8.0, 1.9 Hz), 7.18 (t, 1H, HAr, J = 8.0 Hz), 7.58 (br s, 2H, NH2), 9.46 (s, 1H, OH), 11.06 (s, 2H, 2NH) ppm, 13C NMR (DMSO) δ: 88.5, 98.3, 114.3, 115.2, 115.4, 118.1, 128.8, 137.9, 150.2, 155.4, 156.6, 159.0, 159.8, 160.8. MS: (M+) m/z, 295, 294, 266, 251, 118, 77, 57, 43.
7-amino-2,4-dioxo-5-(4-methoxyphenyl)-1,2,3,4-tetrahydropyrido[2,3-d]pyrimidine-6-carbonitrile (4e).
Brick-red solid, Mp. > 300˚C (Dec.), IR (KBr, cm−1): 3404, 3328 (NH2), 3188, 3150 (2NH), 2219 (CN), 1700, 1645 (2CO). 1H NMR (500 MHz, DMSO): δ 3.80 (s, 3H, OCH3), 6.94 (m, 2H, HAr), 7.19 (m, 2H, HAr), 7.57 (br s, 2H, NH2), 10.59 (s, 1H, NH), 10.73 (s, 1H, NH) ppm, 13C NMR (DMSO) δ: 55.0, 88.8, 98.3, 112.9, 115.7, 128.6, 129.23, 150.1, 155.5, 158.8, 159.3, 160.0, 160.8. MS: (M+) m/z, 309, 265, 121, 77, 57, 43.
7-amino-2,4-dioxo-5-(4-methyphenyl)-1,2,3,4-tetrahydropyrido[2,3-d]pyrimidine-6-carbonitrile (4f).
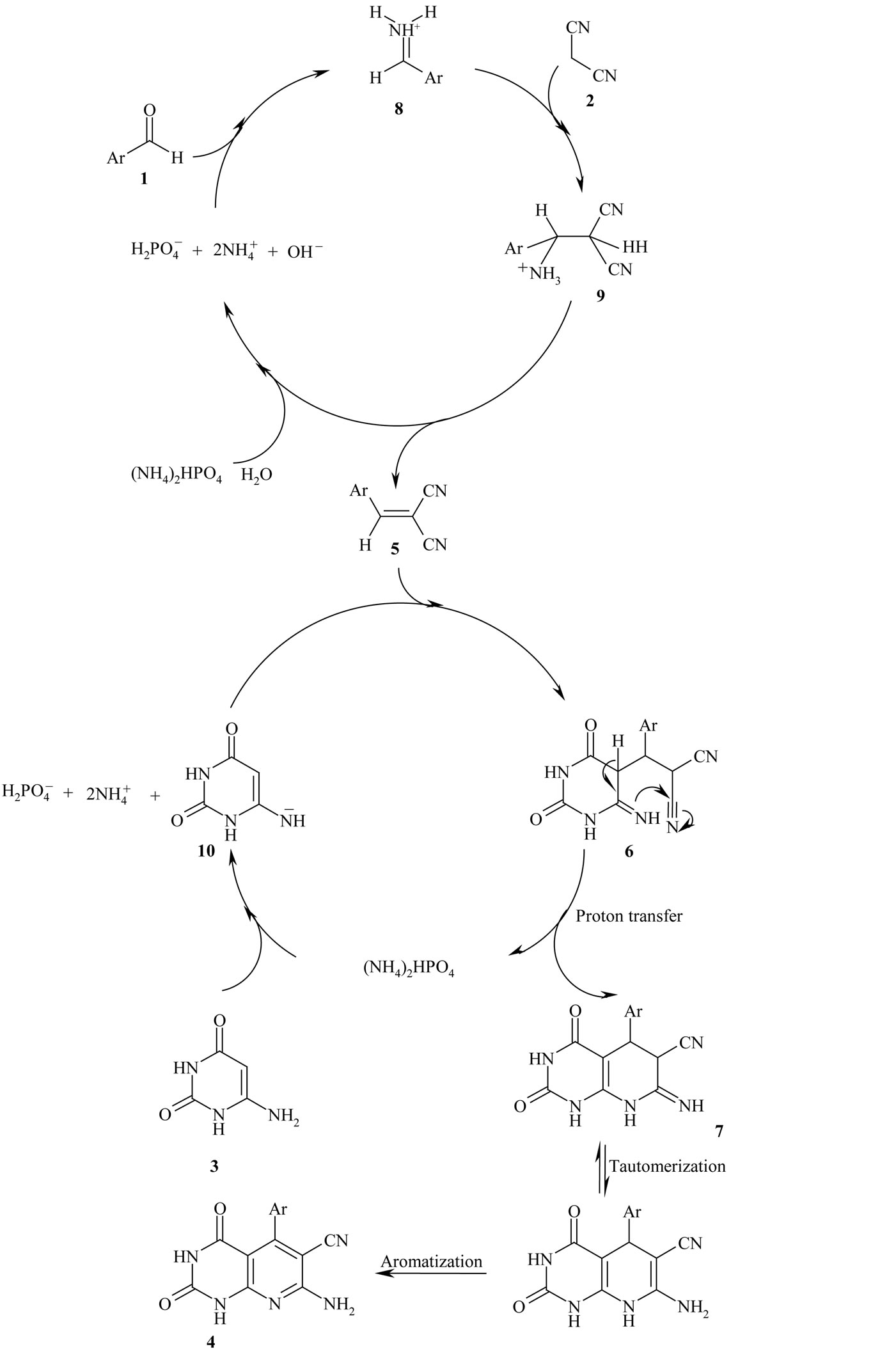
Scheme 3. Suggested mechanism for the synthesis of pyrido[2,3-d]pyrimidines in the presence of DAHP in aqueous media.
White solid, Mp. > 300˚C (Dec.), IR (KBr, cm−1): 3394, 3281 (NH2), 3167, 3031 (2NH), 2222 (CN), 1699, 1645 (2CO). 1H NMR (500 MHz, DMSO): δ 2.36 (s, 3H, CH3), 7.12 (d, 2H, HAr, J = 8.0 Hz), 7.20 (d, 2H, HAr, J = 8.0 Hz), 7.60 (br s, 2H, NH2), 10.89 (s, 1H, NH), 11.43 (s, 1H, NH) ppm, 13C NMR (DMSO) δ: 20.9, 88.7, 98.3, 115.5, 127.5, 128.1, 133.7, 137.4, 150.1, 155.5, 159.1, 159.9, 160.8. MS: (M+) m/z, 293, 292, 249, 77, 57, 43.
7-amino-2,4-dioxo-5-(3-nitrophenyl)-1,2,3,4-tetrahydropyrido[2,3-d]pyrimidine-6-carbonitrile (4g).
Pale yellow solid, Mp. > 300˚C (Dec.), IR (KBr, cm−1): 3384, 3321 (NH2), 3172, 3081 (2NH), 2216 (CN), 1718, 1662 (2CO). 1H NMR (500 MHz, DMSO): δ 7.75 (m, 2H, HAr), 7.77 (br s, 2H, NH2), 8.19 (1H, HAr, J = 1.7 Hz), 8.29 (qd, 1H, HAr, J = 7.0, 1.1 Hz), 11.00 (s, 1H, NH), 11.54 (s, 1H, NH) ppm, 13C NMR (DMSO) δ: 88.3, 98.3, 115.2, 122.8, 123.1, 129.4, 134.4, 138.4, 147.1, 150.1, 155.5, 156.1, 160.2, 160.8. MS: (M+) m/z, 324, 277, 77, 57, 43.
7-amino-2,4-dioxo-5-(4-nitrophenyl)-1,2,3,4-tetrahydropyrido[2,3-d]pyrimidine-6-carbonitrile (4h).
Brickred solid, Mp. > 300˚C (Dec.), IR (KBr, cm−1): 3607, 3534 (NH2), 3297, 3070 (2NH), 2222 (CN), 1703, 1590 (2CO). 1H NMR (500 MHz, DMSO): δ 7.58 (d, 2H, HAr, J = 8.6 Hz), 7.75 (br s, 2H, NH2), 8.27 (d, 2H, HAr, J = 8.6 Hz), 11.00 (s, 1H, NH), 11.55 (s, 1H, NH) ppm, 13C NMR (DMSO) δ: 87.9, 98.2, 115.1, 122.9, 129.2, 144.0, 147.3, 150.2, 155.5, 156.6, 160.1, 160.8, 166.4. MS: (M+) m/z, 324, 277, 77, 57, 43.
6. Acknowledgements
Saeed Balalaie is thankful to Ministry of Science, research, and Technology for financial support.
REFERENCES
- L. V. G. Nargund, Y. S. R. Reddy and R. Jose, “Synthesis and Antibacterial Activity of Pyrido[1, 2-a]pyrimidin-4 (1H)—Ones,” Indian Drugs, Vol. 29, No. 1, 1991, pp. 45- 46.
- A. D. Broom, J. L. Shim and G. L. Anderson, “Pyrido[2,3-d]pyrimidines. IV. Synthetic Studies Leading to Various Oxopyrido[2,3-d]pyrimidines,” Journal of Organic Chemistry, Vol. 41, No. 7, 1976, pp. 1095-1099. doi:10.1021/jo00869a003
- E. M. Grivsky, S. Lee, C. W. Sigel, D. S. Duch and C. A. Nichol, “Synthesis and Antitumor Activity of 2,4-Diamino-6-(2,5-dimethoxybenzyl)-5-methylpyrido[2,3-d]pyrimidine,” Journal of Medicinal Chemistry, Vol. 23, 1980, pp. 327-329. doi:10.1021/jm00177a025
- K. Furukawa and T. Hasegawa, “Preparation of Pyrido[2, 3-d]pyrimidine-2,4-di-one Derivatives as Antiasthmatics and Antiallergics,” Chemical Abstracts, Vol. 124, 1996, 289568c.
- A. Rosowsky, C. E. Mota and S. F. Queener, “Synthesis and Antifolate Activity of 2,4-Diamino-5,6,7,8-tetrahydropyrido[4,3-d]pyrimidine Analogues of Trimetrexate and Piritrexim,” Journal of Heterocyclic Chemistry, Vol. 32, No. 1, 1995, pp. 335-340. doi:10.1002/jhet.5570320155
- A. M. Thompson, A. J. Bridges, D. W. Fry, A. J. Kraker and W. A. Denny, “Tyrosine Kinase Inhibitors.7.7-amino-4-(phenylamino)-and7-amino-4-[(phenylmethyl)amino] pyrido [4,3-d]pyrimidines: A New Class of Inhibitors of the Tyrosine Kinase Activity of the Epidermal Growth Factor Receptor,” Journal of Medicinal Chemistry, Vol. 38, 1995, pp. 3780-3788. doi:10.1021/jm00019a007
- I.O. Donkor, C. L. Klein, L. Liang, N. Zhu, E. Bradley and A. M. Clark, “Synthesis and Antimicrobial Activity of Some 6,7-Annulated Pyrido[2,3-d]pyrimidines,” Journal of Pharmaceutical Sciences, Vol. 84, No. 5, 1995, pp. 661- 664. doi:10.1002/jps.2600840526
- A. Pastor, R. Alajarin, J. J. Vaquero, J. Alvarez-Builla, M. F. d. Casa-Juana, C. Sunkel, J. G. Priego, I. Fonseca and J. Sanz-Aparicio, “Synthesis and Structure of New Pyrido[2,3-d]pyrimidine Derivatives with Calcium Channel Antagonist Activity,” Tetrahedron, Vol. 50, No. 27, 1994, pp. 8085-8098. doi:10.1016/S0040-4020(01)85291-1
- J. Matsumoto and S. Minami, “Pyrido[2,3-d]pyrimidine Antibacterial Agents. 3. 8-Alkyland 8-vinyl-5,8-dihydro-5-oxo-2-(1-piperazinyl)pyrido[2,3-d]pyrimidine-6-carboxylic Acids and Their Derivatives,” Journal of Medicinal Chemistry, Vol. 18, 1975, pp. 74-79. doi:10.1021/jm00235a017
- N. Suzuki, “Synthesis of Antimicrobial Agents. V. Synthesis and Antimicrobial Activities of Some Heterocyclic Condensed 1, 8-Naphthyridine Derivatives,” Chemical & Pharmaceutical Bulletin, Vol. 28, No. 3, 1980, pp. 761- 768. doi:10.1248/cpb.28.761
- V. Oakes and H. N. Rydon, “Polyazanaphthalenes. Part IV. Further Derivatives of 1:3:5- and 1:3:8-triazanaphthalene,” Journal of the Chemical Society, 1956, pp. 4433-4438. doi:10.1039/jr9560004433
- J. I. Degraw, R. L. Kisliuk, Y. Gaumont and C. M. Baugh, “Antimicrobial Activity of 8-Deazafolic Acid,” Journal of Medicinal Chemistry, Vol. 17, No. 4, 1974, pp. 470- 471. doi:10.1021/jm00250a026
- V. E. Kolla, A. B. Deyanov, F. Y. Nazmetdinov, Z. N. Kashina and L. P. Drovosekova, “Investigation of the Anti-Inflammatory and Analgesic Activity of 2-Substituted 1-Aryl-6-carboxy-(carbethoxy)-7-methyl-4-oxo-1,4- dihydropyrido[2,3-d]pyrimidines,” Journal of Pharmaceutical Chemistry, Vol. 27, No. 9, 1993.
- J. W. Ellingboe and N. J. Princeton, “Substituted Pyridopyrimidines and Antihypertensives,” Chemical Abstracts, Vol. 124, 1996, Article ID: 176134q.
- A. Agarwal, R. Ashutosh, N. Goyal, P. M. S. Chauhan and S. Gupta, “ Dihydropyrido [2,3-d]pyrimidines as a New Class of Antileishmanial Agents,” Journal of Bioorganic & Medicinal Chemistry, Vol. 13, No. 24, 2005, pp. 6678-6684.
- I. D. Bystryakova, O. A. Burova, G. M. Chelysheva, S. V. Zhilinkova, N. M. Smirnova and T. S. Safonova, “Synthesis and Biological Activity of Pyridol[2,3-d]pyrimidines,” Journal of Pharmaceutical Chemistry, Vol. 25, No. 12, 1991, pp. 874-876. doi:10.1007/BF00778976
- A. B. Deyanov, R. K. Niyazov, F. Y. Nazmetdivov, B. Y. Syropyatov, V. E. Kolla and M. E. Konshin. “Synthesis and Biological Activity of Amides and Nitriles of 2- Arylamino-5-carboxy(carbethoxy)-6-methylnicotinic acids and 1-aryl-6-carbethoxy-7-methyl-4-oxo-1,4-dihydropyrido[2,3-d]pyrimidines,” Journal of Pharmaceutical Chemistry, Vol. 25, No. 4, 1991, pp. 248-250. doi:10.1007/BF00772106
- A. Monge, V. Martinez-Merino, C. Sanmartin, F. J. Fernandez, M. C. Ochoa, C. Berllver, P. Artigas and E. Fernandez-Alvarez, “2-Arylamino-4-oxo-3,4-dihydropyrido-[2, 3-d]pyrimidines: Synthesis and Diuretic Activity,” European Journal of Medicinal Chemistry, Vol. 24, No. 3, 1989, pp. 24-209. doi:10.1016/0223-5234(89)90001-9
- H. Saladowska, A. Bartoszko-Malik and T. Zawisza, “Synthesis and Properties of New Derivatives of Ethyl 7-Methyl-2,4-dioxo-1,2,3,4-tetrahydropyrido [2,3-d]pyrimidine-5-carboxylate,” Farmaco, Vol. 45, No. 1, 1990, pp. 101-110.
- P. A. Grieco, “Organic Synthesis in Water,” Blackie Academic & Professional, London, 1998.
- C.-J. Li and T. H. Chan, “Comprehensive Organic Reactions in Aqueous Media,” John Wiley &Sons, Inc., Hoboken, 2007.
- C.-J. Li. “Organic Reactions in Aqueous Media with a Focus on Carbon-Carbon Bond Formations: A Decade Update,” Chemical Reviews, Vol. 105, No. 8, 2005, pp. 3095-3166. doi:10.1021/cr030009u
- C.-J. Li, “Organic Reactions in Aqueous Media—With a Focus on Carbon-Carbon Bond Formation,” Chemical Reviews, Vol. 93, No. 6, 1993, pp. 2023-2035. doi:10.1021/cr00022a004
- U. M. Lindström, “Stereoselective Organic Reactions in Water,” Chemical Reviews, Vol. 102, No. 8, 2002, pp. 2751-2772. doi:10.1021/cr010122p
- M. C. Pirrung, “Acceleration of Organic Reactions through Aqueous Solvent Effects,” Chemistry A European Journal, Vol. 12, No. 5, 2006, pp. 1312-1317. doi:10.1002/chem.200500959
- K. V. Katkar, P. S. Chaudhari and K. G.Akamanchi, “Sulfated Tungstate: An Efficient Catalyst for the Ritter Reaction,” Green Chemistry, Vol. 13, No. 4. 2011, pp. 835- 838. doi:10.1039/c0gc00759e
- J. Quiroga, M. Alvarado, B. Insuasty, M. Nogueras, A, Sanchez and J. Cobo, “Synthesis of 6-Cyanopyrido- [2,3-d]pyrimidinones in the Reaction of 6-Amino-4-pyrimidinones with Arylidene Derivatives of Malonodinitrile,” Journal of Heterocyclic Chemistry, Vol. 35, No. 6, 1998, pp. 1309-1311. doi:10.1002/jhet.5570350612
- M. N. Nasr and M. M. Gineinah, “Pyrido[2,3-d]pyrimidines and Pyrimido[5′,4′:5,6]pyrido [2,3-d]pyrimidines as New Antiviral Agents: Synthesis and Biological Activity,” Journal of Heterocyclic Compounds, Vol. 33, No. 50, 2002, p. 118.
- X.-S. Wang, Z.-S. Zeng, D.-Q. Shi, X.-Y. Wei and Z.-M. Zong, “KF-Alumina Catalyzed One-Pot Synthesis of Pyrido[2,3-d]Pyrimidine Derivatives,” Synthetic Communications, Vol. 34, No. 23, 2004, pp. 4331-4338. doi:10.1081/SCC-200039392
- X.-S. Wang, Z.-S Zeng, D.-Q. Shi, S.-J. Tu, X.-Y. Wei and Z.-M. Zong, “Three-Component, One-Pot Synthesis of Pyrido[2,3-d]pyrimidine Derivatives Catalyzed by KF-Alumina,” Synthetic Communications, Vol. 35, No. 14, 2005, pp. 1921-1927. doi:10.1081/SCC-200064984
- S. Youssif and F. Z.Agili, “ChemInform Abstract: OnePot Synthesis of Fused 2-Thiouracils: Pyrimidopyrimidines, Pyridopyrimidines and Imidazolopyrimidines,” Journal of Preparative Organic Chemistry, Vol. 39, No. 43, 2008.
- D. Shi, S. Ji, L. Niu, J. Shi and X. Wang, “One-Pot Synthesis of Pyrido[2,3-d]pyrimidines via Efficient ThreeComponent Reaction in Aqueous Media,” Journal of Heterocyclic Chemistry, Vol. 44, No. 5, 2007, pp. 1083- 1090. doi:10.1002/jhet.5570440517
- D-Q. Shi, Y. Zhou and H. Liua, “An Efficient Synthesis of Pyrido[2,3-d]pyrimidine Derivatives in Ionic Liquid,” Journal of Heterocyclic Chemistry, Vol. 47, No. 1, 2010, pp. 131-135.
- A. Loupy, “Microwave in Organic Synthesis,” WileyVCH, Weinheim, 2002, pp. 147-180. doi:10.1002/3527601775.ch5
- R. S. Varma, “Solvent-Free Organic Syntheses. Using Supported Reagents and Microwave Irradiation,” Green Chemistry, Vol. 1, No. 1, 1999, pp. 43-55. doi:10.1039/a808223e
- C. O. Kappe, “Controlled Microwave Heating in Modern Organic Synthesis,” Angewandte Chemie International Edition, Vol. 43, No. 46, 2004, pp. 6250-6284. doi:10.1002/anie.200400655
- B. L. Hayes, “Recent Advances in Microwave-Assisted Synthesis,” Aldrichimica Acta, Vol. 37, No. 2, 2004, pp. 66-77.
- R. J. Lewis, “Hawley’s Condensed Chemical Dictionary,” 13th Edition, Von Nostrand Reinhold, New York, 1997.
- S.-J. Tu, B. Jiang, R.-H. Jia, J.-Y. Zhang, Y. Zhang, C.-S. Yao and F. Shi, “An Efficient One-Pot, Three-Component Synthesis of Indeno[1,2-b]quinoline-9,11(6H,10H)- dione, Acridine-1,8(2H,5H)-dione and Quinoline-3-carbonitrile Derivatives from Enaminones,” Organic & Biomolecular Chemistry, Vol. 4, No. 16, 2006, pp. 3664- 3668. doi:10.1039/b607575d
- S.-J. Tu, B. Jiang, J.-Y. Zhang, R.-H. Jia, Y. Zhang and C.-S. Yao, “Efficient and Direct Synthesis of Poly-Substituted Indeno[1,2-b]quinolines Assisted by P-Toluene Sulfonic Acid Using High-Temperature Water and Microwave Heating via One-Pot, Three-Component Reaction,” Organic & Biomolecular Chemistry, Vol. 4, No. 21, 2006, pp. 3980-3985. doi:10.1039/b611462h
- S. Balalaie, M. Bararjanian, S. Hekmat and P. Salehi, “Novel, Efficient, and Green Procedure for the Knoevenagel Condensation Catalyzed by Diammonium Hydrogen Phosphate in Water,” Synthetic Communications, Vol. 36, No. 17, 2006, pp. 2549-2557. doi:10.1080/00397910600781471
- F. Darvich, S. Balalaie, F. Chadegani and P. Salehi, “Diammonium Hydrogen Phosphate as a Neutral and Efficient Catalyst for Synthesis of 1,8-Dioxo-octahydroxanthene Derivatives in Aqueous Media,” Synthetic Communications, Vol. 37. No. 7, 2007, pp. 1059-1066. doi:10.1080/00397910701196520
- S. Balalaie, M. Bararjanian, S. Hekmat, M. SheikhAhmadi and P. Salehi, “Diammonium Hydrogen Phosphate: An Efficient and Versatile Catalyst for the One-Pot Synthesis of Tetrahydrobenzo[b]pyran Derivatives in Aqueous Media,” Synthetic Communications, Vol. 37, No. 7, 2007, pp. 1097-1108. doi:10.1080/00397910701196579
- S. Abdolmohammadi and S. Balalaie, “Novel and Efficient Catalysts for the One-Pot Synthesis of 3,4-Dihydropyrano[c]chromene Derivatives in Aqueous Media,” Tetrahedron Letters, Vol. 48, No. 18, 2007, pp. 3299- 3303. doi:10.1016/j.tetlet.2007.02.135
- S. Balalaie, S. Abdolmohammadi, H. R. Bijanzadeh and A. M. Amani, “Diammonium Hydrogen Phosphate as a Versatile and Efficient Catalyst for the One-Pot Synthesis of Pyrano[2,3-d]pyrimidinone Derivatives in Aqueous Media,” Molecular Diversity, Vol 12, No. 2, 2008, pp. 85-91. doi:10.1007/s11030-008-9079-7
NOTES
*Corresponding author.

