International Journal of Clinical Medicine
Vol.2 No.2(2011), Article ID:5106,5 pages DOI:10.4236/ijcm.2011.22028
Risperidone Versus Yokukansan in the Treatment of Severe Alzheimer’s Disease
![]()
1Medical Care Center, Shizuoka University, Shizuoka City, Japan; 2Internal Medicine, Asahi Hospital, Sayama City, Japan.
Email: oyfuruh@ipc.shizuoka.ac.jp
Received February 9th, 2011; revised April 12th, 2011; accepted April 15th, 2011.
Keywords: Alzheimer’s Disease, Behavioral and Psychological Symptoms of Dementia, Dementia
ABSTRACT
Purpose: Patients with AD commonly exhibit behavioral and psychological symptoms of dementia (BPSD). This study is aimed to compare the efficacy of yokukansan (YKS) and risperidone (RIS) on BPSD in patients with severe Alzheimer’s disease (AD). Methods: Thirty-eight inpatients with AD were investigated. Patients were randomly assigned to the YKS group (N = 18) or the RIS group (N = 20) and treated for 4 weeks. The primary outcomes were changes in the scores on the Neuropsychiatric Inventory (NPI), the Mini-Mental State Examination (MMSE), the Barthel Index, and the Cohen-Mansfield Agitation Inventory (CMAI). The frequency of extrapyramidal symptoms (EPS) and other adverse events were recorded at every visit. Results: All participants in both groups completed the trial. The Barthel Index did not significantly change either in the RIS group or the YKS group. The MMSE scores did not change either in the RIS group or the YKS group. Significant improvements in mean total NPI and CMAI scores showed in both groups. Between the YKS and the RIS groups, there were no significant differences in the NPI or the CMAI scores. EPS and other serious adverse effects were not observed in either group. Conclusions: In this 4-week trial, YKS treatment significantly improved BPSD in the patients with severe AD. The present study suggests that YKS is as effective as RIS on BPSD with severe AD.
1. Introduction
Alzheimer’s disease (AD) is a progressive neurodegenerative disorder that presents with deterioration of memory and cognitive function, progressive impairment of daily activities, and many neuropsychiatric impairments [1]. Patients with AD commonly exhibit behavioral and psychological symptoms of dementia (BPSD). When BPSD are moderate to severe, an appropriate pharmacological treatment is needed. While there have been several studies of pharmacological interventions for BPSD, a single strategy for treatment of BPSD has not been sufficiently supported [2-4].
The utility of risperidone in patients with BPSD has been evaluated in two placebo-controlled, double-blind clinical trials [5,6]. Furthermore, the study of long-term trials reported that low-dose risperidone was effective and well tolerated in the treatment of BPSD [7]. On the other hand, quetiapine did not significantly improve psychosis scores despite good compliance and a reasonable time to attain treatment [8].
Recently, yokukansan (YKS), the traditional Japanese medicine, which has been used for the treatment of neurosis, insomnia, and irritability in infants, was reported to improve BPSD without any serious adverse events, [9- 13]. However, most of the patients in these reports were included dementia other than AD, and the levels of cognitive function assessed with the Mini-Mental State examination (MMSE) [14] were mild to moderate.
There is no report that directly compares efficacy and safety of risperidone (RIS) and YKS in elderly patients with severe AD. This study aims to compare the efficacy and safety of YKS and RIS in the improvement of BPSD in patients with severe AD.
2. Materials and Methods
2.1. Subjects
All subjects were recruited from Asahi Hospital, a longterm care facility located in Saitama prefecture, Japan.
The diagnosis of dementia was made in accordance with the Diagnosis and Statistical Manual of Mental Disorder, Fourth Edition (DSM-Ⅳ) criteria [15]. The diagnosis of AD was made in accordance with the National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer’s Disease and Related Disorders Association (NINCDS-ADRDA) criteria [16]. The inclusion criteria were 1) diagnosis of probable AD as defined by the NINCDS-ADRDA, 2) an MMSE score of 12 or less, 3) presence of at least one BPSD symptom, and 4) stable physical condition for at least three months. The exclusion criteria were 1) with the presence of psychiatric or neurological disorders, 2) the use of concomitant medications that have primarily central nervous system activity including, cholinesterase inhibitors, anticonvulsants, mood stabilizers, antipsychotics other than risperidone, antidepressants, and Chinese herbal medicines other than YKS, 3) presence of other types of dementia, 4) presence of acute disorders, such as infection, heart failure, or stroke, requiring special treatment and intensive care that might affect BPSD.
After a detailed explanation of the study, we obtained written informed consent from the families of all patients. Patients were randomly assigned to the RIS group or the YKS group.
2.2. Intervention
At baseline, each patient received a uniform evaluation that included a medical history, a physical and neurological examination, and a brain magnetic resonance (MRI). YKS, manufactured by Tsumura & Co. (Tokyo, Japan) and approved for ethical use by the Ministry of Health, Labor, and Welfare of Japan, is a dried extract of the following raw materials: 4.0 parts Atracylodis Lanceae Rhizoma (rhizome of Atracylodes lancea De Candolle, Compositae), 4.0 parts Poria (scierotium of poria cocos Wolf, polyporaceae), 3.0 parts Cnidii Rhizoma (rhizome of Cnidium officinale Makino, Umbelliferae), 3.0 parts Angelicae Radix (root of Angelica acutiloba Kitagawa, Umbelliferae), 2.0 parts Bupleuri Radix (root of Bupleurum falcatum Linne, Umbelliferae), 1.5 parts Glycyrrhizae Radix (root and stolon of glycyrrhiza uralensis Fisher, Leguminosae),and 3.0 parts Uncaria Uncis Cum Ramulus (hook of Uncaria rhynchophilla Miquel, Rubiaceae).
The patients in the YKS group received 7.5 g of YKS three times every day before meals. Patients in the RIS group received 1 mg of RIS once daily at bedtime. Patients were treated with YKS or RIS for four weeks.
2.3. Measurements
Neurocognitive functioning was evaluated by the change from baseline (Week 0) to completion (Week 4) in the MMSE. The MMSE is used to detect and follow the progression of cognitive impairment associated with neurodegenerative disorders. Scores range from 0 to 30, with higher scores indicating better performance. Activities of daily living (ADL) were evaluated by the change from Week 0 to Week 4 in the Barthel Index [17]. The Barthel Index is a scale used to measure performance in basic ADL. The 10-item version of the instrument was used. Scores range from 0 to 100, with higher scores indicating better performance.
Efficacy measures were evaluated by the change from Week 0 to Week 4 in the Neuropsychiatric Inventory (NPI) [18] and the Cohen-Mansfield Agitation Inventory (CMAI) [19]. The NPI has 12 neuropsychiatric domains. Scores range from 1 to 144, with higher scores indicating more frequent or severe symptoms and signs. The CMAI includes 29 items. Scores range from 29 to 203, with higher scores indicating more frequent or severe agitation. Scores above 40 are usually considered to be clinically significant.
Safety was assessed at every visit by recording adverse events (AEs), routine physical examinations (including body weight), vital signs (blood pressure, pulse, and temperature). In addition, general conditions including bowel movement, amount of food ingested, and sleeping condition were recorded at every visit. Electrocardiograms (ECG) and laboratory tests (chemistry, electrolytes, hematology, serum potassium, and urinalysis) were conducted at Week 0 and Week 4. AEs potentially associated with antipsychotics were analyzed as well and included edema-related events, glucose related events, extrapyramidal symptoms (EPS), and cardiovascular events.
2.4. Statistical Analysis
After the completion of the treatment periods, patients in both the YKS and the RIS groups were reassessed by using the Barthel Index, MMSE, NPI, and CAMI. For non-normally distributed outcomes, we performed nonparametric tests. The results were analyzed by using SPSS version 12 (SPSS Inc, Chicago, IL). Demographic data were analyzed using mean and standard deviation (SD). Intra-group comparisons preand post-treatment data (0 W versus 4 W) were analyzed non-parametrically using the Wilcoxon’s signed-rank test. Mann-Whitney U-tests or χ2 tests were used for comparisons between the YKS and the RIS groups, as appropriate. The criterion for statistical significance was considered to be p < 0.05.
3. Result
We included 38 patients who were judged appropriate for the study. The demographic data of the patients are shown in Table 1. No significant differences between the YKS group and the RIS group were detected with respect to age, gender, weight, the Barthel Index, the MMSE scores, the NPI scores, or the CAMI scores.

Table 1. Demographic and baseline characteristics of patients.
Intra-group comparisons (Week 0 versus Week 4), the significant improvement in the mean NPI score was observed both in the YKS group (p = 0.000, the Wilcoxson’s signed-rank test) and in the RIS group (the p = 0.000, the Wilcoxson’s signed-rank test). The significant improve ment in the mean CMAI score was observed both in the YKS group (p = 0.000, the Wilcoxson’s signed-rank test) and in the RIS group (p = 0.000, the Wilcoxson’s signed-rank test). Between the YKS and the RIS groups (the YKS group versus the RIS group), there was no significant difference in the NPI scores (p = 0.92, the Mann-Whitney test). In CMAI scores, there was no significant difference (p = 0.75, the Mann-Whitney test) (Table 2). These results showed no significant differences between the YKS and the RIS in efficacy.
After four weeks of treatment with YKS or RIS, the Barthel Index did not significantly change either in the YKS group or in the RIS group. The MMSE scores did not change significantly within the two groups (Table 2). The MMSE and the Barthel Index scores remained stable from baseline to Week 4 on individual patient-level.
Throughout the duration of the study, no clinically meaningful changes including vital signs, laboratory data, and ECG were shown either in the YKS group or in the RIS group (Table 3). Extrapyramidal signs were not observed either in the YKS group or in the RIS group. Adverse events (AEs) reported in one patient (5.6%) in the YKS group and three patients (15%) in the RIS (Table 4).
In the occurrence of AEs, the RIS group had significantly higher rates than the YKS group (p = 0.029, χ2 test).
4. Discussion
In this study, we compared the efficacy and safety of YKS versus RIS in patients with severe AD. This study suggests that YKS is as effective as low-dose RIS in the treatment of BPSD with severe AD. In addition, YKS
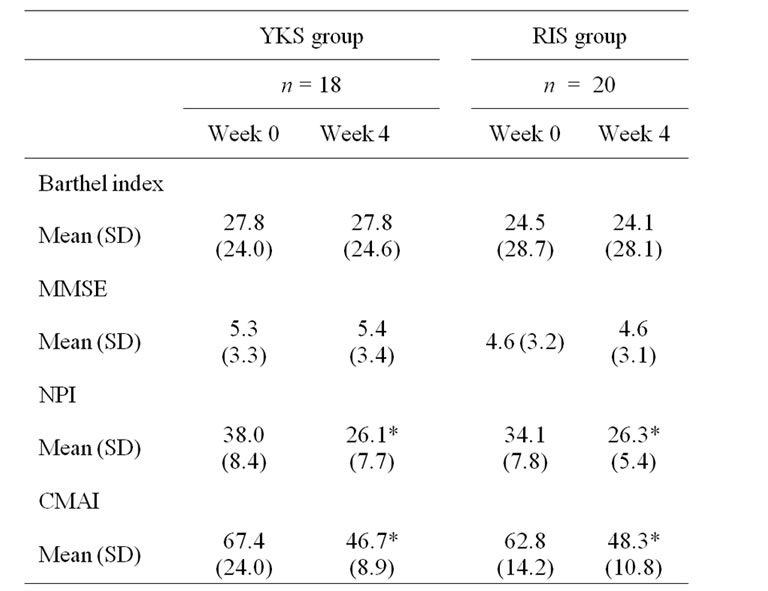
Table 2. Scores of the Barthel Index, the MMSE, the NPI, and the CMAI at Week 0 and Week 4 in the YKS and the RIS group.

Table 3. Main data of vital signs and laboratory tests of the YKS and the RIS groups at Week 0 and Week 4.

Table 4. Adverse events in the two treatment groups.
does not cause either cognitive decline or ADL decline and causes no serious adverse effects, which is in line with previous studies [10-13]. Furthermore, this study shows that YKS is safer than RIS in the occurrence of AEs. Regarding the safety of YKS, however, some studies reported that YKS might cause hypokalemia [11,13]. In this study, serum potassium levels did not change significantly (Table 3: 4.05 ± 0.26 mEq/L to 4.01 ± o.26 mEq/L) and serum levels in all patients remained within normal range, there should be a tendency that potassium levels reduce according to the administration of YKS. It is necessary to perform the relevant tests during the treatment with YKS.
Recently, several prior studies have investigated the mechanism of action of YKS. Fujiwara et al. [20] demonstrated that Uncaria rhynchophylla, a constituent herb of YKS, has a potent anti-aggregation effect on beta-amyloid (Aβ) proteins in vitro. The other study reports that YKS has an effect on Aβ induced cytotoxicity on rat cortical neurons [21]. The details of the mechanisms should be investigated in future studies.
These results suggest that YKS might be instrumental in the amelioration of the dysfunction in various neuronal systems caused by Aβ, which is thought to be a causative substance of AD.
Furthermore, YKS was demonstrated to suppress the excessive release of glutamate and aspartate in the hippocampus of zinc-deficient rats [22] and to decrease expression of 5-hydroxytruptamine (5-HT) receptors in the prefrontal cortex of rats [23].
In addition, it has been reported that YKS ameliorates the impairment of learning ability, deficits of normal anxiety, and loco motor hyperactivity in APP-Tg (+) mice, which is considered to be an animal model of Alzheimer’s disease [24].
5. Conclusions
These findings suggest that YKS possesses multiple biological effects. YKS is not a simple preparation of medicine, as it contains many ingredients, and the interactions between ingredients can be important. The details of the mechanisms should be investigated in future studies.
Although the present study included only a small number of patients and only four weeks of the YKS treatment, YKS may be a new avenue of treatment for BPSD in the patients with severe AD.
A double-blind, placebo-controlled study, which includes a larger patient population and a longer duration of YKS treatment will be necessary to conform the efficacy and safety of YKS for BPSD in severe AD.
REFERENCES
- R. Howard, C. Ballard , J. O’Brien, A. Burns and UK and Ireland Group for Optimization of Management in dementia, “Guidelines for the Management of Agitation in Dementia,” International Journal of Geriatric Psychiatry, Vol. 16, No. 7, 2001, pp. 714-717. doi:10.1002/gps.418
- P. E. Lee, S. S. Gill, M. Freedman , S. E. Bronskill, M. P. Hillmer and P. A. Rochon, “Atypical Antipsychotic Drugs in the Treatment of Behavioral and Psychological Symptoms of Dementia: Systematic Review,” British Medical Journal, Vol. 329, No. 10, 2004, pp. 7457-7475.
- L. S. Schneider, P. N. Tariot, K. S. Dagerman , S. M. Davis, J. K .Hsiao, M. S. Ismail, B. D. Lebowitz, C. G. Lyketsos, J. M. Ryan, T. S. Stroup, D. L. Sultzer, D. Weintraub, J. A. Lieberman and CATIE-AD Study Group, “Effectiveness of Atypical Antipsychotics Drugs in Patients with Alzheimer’s Disease,” The New England Journal of Medicine, Vol. 355, No. 15, 2006, pp. 1525 -1538.
- P. Rocca, F. Marino, C. Montemagni, D. Perrone and F. Bogetto, “Risperidone, Olanzapine and Quetiapine in the Treatment of Behavioral and Psychological Symptoms in Patients with Alzheimer’s Disease: Preliminary Finding from a Naturalistic, Retrospective Study,” Psychiatry Clinical Neuroscience, Vol. 61, No. 6, 2007, pp. 622-629. doi:10.1111/j.1440-1819.2007.01729.x
- P. P. De Deyn, K. Rabheru, A. Rasmussen, J. P. Bocksberger, P. L. Dautzenberg, S. Eriksson and B. A. Lawlor, “A Randomized Trial of Risperidone, Placebo, and Haloperidol for Behavioral Symptoms of Dementia,” Neurology, Vol. 53, No. 5, 1999, pp. 946-595.
- I. R. Katz, D. V. Jeste, J. E. Mintzer, C. Clyde, J. Napolitano and M. Brecher, “Comparison of Risperidone and Placebo for Psychosis and Behavioral Disturbances Associated with Dementia: A Randomized, Double-Blind Trial,” Journal of Clinical Psychiatry, Vol. 60, No. 2, 1999, pp. 107-115. doi:10.4088/JCP.v60n0207
- P. P. De Deyn and I. R. Katz, “Control of Aggression and Agitation in Patients with Dementia: Efficacy and Safety of Risperidone,” International Journal of Geriatric Psychiatry, Vol. 15, No. 1, 2000, pp. 514-522. doi:10.1002/1099-1166(200007)15:1+<::AID-GPS168>3.0.CO;2-#
- D. Paleacu, Y.Barak, I. Mirecky and D. Mazeh, “Quetiapine Treatment for Behavioural and Psychological Symptoms of Dementia in Alzheimer’s Disease Patients: A 6-Week, Double-Blind, Placebo-Controlled Study,” International Journal of Geriatric Psychiatry, Vol. 23, No. 4, 2008, pp. 393-400. doi:10.1002/gps.1892
- K. Iwasaki, T. Satoh-Nakagawa, M. Maruyama, Y. Monma, M. Nemoto, N. Tomita, H. Tanji, H. Fujiwara, T. Seki, M. Fujii, H. Arai and H. Sasaki, “A Randomized, Observer-Blind, Controlled Trial of the Traditional Chinese Medicine Yi-Gan San for Improvement of Behavioral and Psychological Symptoms and Activities of Daily Living in Dementia Patients,” Journal of Clinical Psychiatry, Vol. 66, No. 2, 2005, pp. 248-252. doi:10.4088/JCP.v66n0214
- H. Shinno, E. Utani, S. Okazaki, T. Kawamukai, H. Yasuda, T. Inagaki, Y. Inami and J.Horiguchi, “Successful Treatment with Yi-Gan San for Psychosis and Sleep Disturbances in a Patient with Dementia with Lewy Bodies,” Progress in Neuro-Psychopharmacology & Biological Psychiatry, Vol. 31, No. 7, 2007, pp. 1543-1545. doi:10.1016/j.pnpbp.2007.07.002
- K. Mizumaki, “Kampo Therapy as an Alternative to Pharmacotherapy Using Antipsychotic Medicines for Behavioral and Psychological Symptoms of Dementia (BPSD),” Psychogeriatrics, Vol. 8, No. 3, 2008, pp. 137-141. doi:10.1111/j.1479-8301.2008.00242.x
- H. Shinno, Y. Inami, T. Inagaki, Y. Nakamura and J. Horiguchi, “Effect of Yi-Gan San on Psychiatric Symptoms and Sleep Structure at Patients with Behavioral and Psychological Symptoms of Dementia,” Progress in Neuro-Psychopharmacology & Biological Psychiatry, Vol. 32, No. 3, 2008, pp. 881-885. doi:10.1016/j.pnpbp.2007.12.027
- K. Mizukami, T. Asada, T. Kinoshita, K. Tanaka, K. Sonohara, R. Nakai, K. Yamaguchi, H. Hanyu, K. Kanaya, T. Takao, M. Okada, S. Kudo, H. Kotoku, M. Iwaskiri, H. Kurita, T. Miyamura, Y. Kawasaki, K. Omori, K. Shiozaki, T. Odawara, T. Suzuki, S. Yamada, Y. Nakamura and K. Toba, “A Randomized Cross-over Study of a Traditional Japanese Medicine (Kampo), Yokukansan, in the Treatment of the Behavioral and Psychological Symptoms of Dementia,” International Journal of Neuropsychopharmacoly, Vol. 12, No. 2, 2009, pp. 191-199. doi:10.1017/S146114570800970X
- M. F. Folstein, S. E. Folstein and P. R. McHugh, “Mini Mental State: A Practical Method for Grading the Cognitive State of Patients for the Clinician,” Journal of Psychiatric Research, Vol. 12, No. 3, 1975, pp. 189-198. doi:10.1016/0022-3956(75)90026-6
- American Psychiatric Association, “Diagnostic and Statistical Manual of Mental Disorders” 4th Edition, American Psychiatric Press, Washington D.C., 1994.
- G. Mckhann, D. Drachman, M. Folstein, R. Katzman, D.Price and E. M. Stadlan, “Clinical Diagnosis of Alzheimer’s Disease: Report of the NINCDS-ADRDA Work Group under the Auspices of the Department of Health and Human Services Task Force on Alzheimer’s Disease,” Neurology, Vol. 34, No. 7, 1984, pp. 939-944.
- F. I. Mahoney and D. W. Barthel, “Functional Evaluation: The Barthel Index,” Maryland State Medical Journal, Vol. 14, 1965, pp. 61-65.
- J. L. Cummings, M. Mega, K. Gray, S. RosenbergThompson, D. A. Carusiand and J. Gornbein, “The Neuropsychiatric Inventory: Comprehensive Assessment of Psychopathology in Dementia,” Neurology, Vol. 44, No. 12, 1994, pp. 2308-2314.
- J. Cohen-Mansfield, M. S. Mark and A. S. Rosenthal, “A Description of Agitation in a Nursing Home,” Journal of Gerontology, Vol. 44, No. 3, 1989, pp. 77-84.
- H. Fujisawa, K. Iwasaki, K. Furukawa, T. Seki, M. He, M. Maruyama, N. Tomita, Y. Kudo, M. Higuchi, T. C. Saido, S. Maeda, A.Takashima, M.Hara, Y. Ohizumi and H. Arai, “Uncaria Rhynochophylla, a Chinese Medical Herb, Has Potent Anti-Aggregation Effects on Alzheimer’S Beta-Amyloid Proteins,” Journal of Neuroscience Research, Vol. 84, No. 2, 2006, pp. 427-433.
- M. Tateno, W. Ukai, T. Ono, S. Saito, E. Hashimoto and T. Saito, “Neuroprotective Effects of Yi-Gan San against Beta Amyloid-Induced Cytotoxicity on Rat Cortical Neurons,” Progress in Neuro-Psychopharmacology & Biological Psychiatry, Vol. 32, No. 7, 2008, pp. 1704-1707. doi:10.1016/j.pnpbp.2008.07.006
- A. Takeda, H. Itoh, H. Tamano, M.Yuzurihara and N. Oku, “Suppressive Effects of Yokukansan on Excessive Release of Glutamate and Aspartate in Hippocampus of Zinc-Deficient Rats,” Nutritional Neuroscience, Vol. 11, No. 1, 2008, pp. 41-46. doi:10.1179/147683008X301414
- N. Egashira, K. Iwasaki, A. Ishibashi, K. Hayakawa, R. Okuno, M. Abe, N. Uchida, K.Mishima, K. Takasaki, R. Nishimura, R. Oishi and M. Fujiwara, “Repeated Administration of Yokukansan Inhibits DOI-Induced Head-Twitch Response and Decreases Expression of 5-Hydroxy-Truptamine (5-HT)2A Receptors in the PreFrontal Cortex,” Progress in Neuro-Psychopharmacology & Biological Psychiatry, Vol. 32, No. 6, 2008, pp. 1516-1520. doi:10.1016/j.pnpbp.2008.05.010
- M. Tabuchi, T. Yamaguchi, S. Iizuka, S. Imamura, Y.Ikarashi and Y. Kase, “Ameliorative Effects of YokuKansan, a Traditional Japanese Medicine, on Learning and Non-Cognitive Disturbances in the Tg2576 Mouse Model of Alzheimer’s Disease,” Journal of Ethnopharmacology, Vol. 122, No. 1, 2009, pp. 157-162. doi:10.1016/j.jep.2008.12.010

