Open Journal of Preventive Medicine
Vol.2 No.3(2012), Article ID:21562,8 pages DOI:10.4236/ojpm.2012.23053
Glycemic and cardiovascular parameters improved in type 2 diabetes with the high nutrient density (HND) diet
![]()
1Private Practice, Medical Compass MD, East Setauket, USA
2Private Practice, Flemington, USA; *Corresponding Author: mdoffice@drfuhrman.com
3F.M. Kirby Center for Molecular Ophthalmology, University of Pennsylvania, Philadelphia, PA, USA
4Center for Preventive Ophthalmology and Biostatistics, Perelman School of Medicine, University of Pennsylvania, Philadelphia, USA
Received 6 April 2012; revised 26 May 2012; accepted 8 June 2012
Keywords: Type 2 Diabetes Mellitus; Lipids; Hypertension; Nutrition; Cardiovascular Disease; Lifestyle Medicine; HbA1C; BMI
ABSTRACT
Objective: The purpose of this study was to provide an initial assessment of the effectiveness of the high nutrient density (HND) diet on glycemic control and cardiovascular risk factors in participants with type 2 diabetes. Design: This was a retrospective case series study. Participants were 13 adult type 2 diabetic U.S. women and men between the ages of 30 - 80 years old. Glycosylated hemoglobin (HbA1C), lipid profile, blood pressure, BMI, and medication requirements before and after commencement of the HND diet were compared. Results: After a median length on the HND diet of 7 months, the mean HbA1C dropped from 8.2% to 5.8% (p = 0.002), with sixty-two percent of participants reaching normoglycemic levels (HbA1C < 6.0%). There was a substantial reduction in mean blood pressure for hypertensive participants (n=10) from a pre-intervention level of 148/87 mmHg to 121/74 mmHg (p = 0.0004 for systolic blood pressure, p = 0.01 for diastolic blood pressure). Triglycerides significantly decreased from a mean of 171 mg/dl to a mean of 103 mg/dl (p = 0.02). The mean HDL increased significantly from 48.3 mg/dl to 54.6 mg/dl (p = 0.03). The mean number of medications dropped from 4 to 1 (p = 0.0006). Conclusions: The HND diet was very effective in controlling glycemic levels and cardiovascular risk factors in 13 participants with type 2 diabetes. Therefore, there is a well-justified need for further study with the HND diet.
1. INTRODUCTION
Lowering complication risk and achieving better metabolic control are the central goals of medical care for type 2 diabetes, but outcomes are inconsistent. In the 2009 consensus statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes, the organizations recommend starting a nascent type 2 diabetes patient on lifestyle changes plus metformin. According to the authors, for most individuals, lifestyle interventions fail to achieve or maintain the metabolic goals [1]. Only about 37% of type 2 diabetes patients in 1999-2000 Third National Health and Nutrition Examination Survey have achieved the ADA’s recommended goal of a HbA1C < 7.0% [2].
Diets low in animal protein and saturated fat and high in complex carbohydrates, fiber and micronutrients improve glucose tolerance, postprandial glucose and overall glycemic control, as well as decrease insulin resistance [3-7]. The high nutrient density (HND) diet emphasizes micronutrients (phytochemicals and antioxidants) from greens, fruits, nuts/seeds and beans/legumes, the latter containing high amounts of viscous fiber and resistant starches. The HND diet incorporates features of other dietary interventions, but is designed to create advantages from multiple mechanisms, including the effect that high micronutrient food has in reducing cravings and overeating, and on lowering oxidative stress [8] and deposition of advanced glycation end products [9,10].
The HND diet is a plant-rich diet differing from other plant-based diets in some subtle, but significant, ways. Foods are rated based on total micronutrient content per calorie, which emphasizes consumption of greens and other non-starchy vegetables, such as onions, mushrooms, eggplant, peppers, tomatoes, and cauliflower, in unlimited quantity. High glycemic, high carbohydrate foods are reduced, while beans, peas, squash and intact grains are permitted. Nuts and seeds are the primary source of fat, while animal products are limited to 10 percent of calories or less. Basic recommendations include:
1) At least one large green salad a day, with inclusion of a nut/seed derived salad dressing.
2) One bowl of vegetable-bean soup daily.
3) 1 - 2 ounces of raw seeds and nuts daily (usually in salad dressing recipe).
4) Approximately 3 - 4 fresh fruits per day.
5) One large serving of steamed or stewed greens, with mushrooms, onions and other low-starch veggies.
6) Only one serving a day of non-bean starch, such as squash, steel cut oats, brown/wild rice.
7) Exclusion of white flour, sweets, and oils, while limiting animal products to 12 ounces per week.
The Mediterranean diet, which has been shown to reduce the risk of type 2 diabetes, encourages moderate amounts of fish and dairy, including fat and non-fat varieties, and emphasizes whole grains and extra virgin olive oil [11-13]. The HND diet, in contrast, suggests only small amounts of fish, non-fat dairy [14], whole grains and minimal amounts or no oil. The HND diet encourages more calories derived from vegetables and beans, as well as the intake of a moderate amount of fats from sources, such as nuts, which contain a combination of protein, fiber, phytonutrients, antioxidants and omega 3 fatty acids [15]. Tree nuts and peanut butter reduce the risk of developing diabetes in women [16,17]. Nuts significantly reduce the risk of coronary heart disease (CHD) and the risk of death from CHD [18-22] in type 2 diabetes patients.
The combination of the plant-based micronutrients recommended in the HND diet has the potential to improve results over those obtained in other medical nutrition studies. To provide an initial evaluation of the potential efficacy of the HND diet in participants with type 2 diabetes and to provide pilot data for large randomized clinical trials of the HND diet, we report a retrospective case series examining multiple parameters in 13 participants with type 2 diabetes, both before and after initiation of the HND diet.
2. METHODS
There were multiple inclusion criteria for this retrospective case series. The cases were selected based on two main criteria. First they needed to have a diagnosis of type 2 diabetes with documented baseline HbA1C readings before starting the HND diet. Second, the participants had to participate in phone interviews tracking their ongoing diet and historical dietary recall. As a result of these phone interviews, documented compliance with the dietary intervention of ≥90% was required for inclusion. Greater than 90% compliance was defined as ≤2 meals per week inconsistent with the HND diet and this minimum standard of consistency was necessary during the entire period of result tracking. The participants were not told of the compliance as an inclusion criterion until after analysis of their dietary intake was completed. Selection also required participants to fall into one of the following two categories during the preintervention period: they had a HbA1C > 7.0% with or without diabetes medications (n = 9); or they had a HbA1C > 6.0% with diabetes medications (n = 4). The participants were from two sources, Dr. Fuhrman’s practice or Dr. Fuhrman’s interactive website.
Dr. Fuhrman was not the primary care physician for these participants, but rather a specialist with expertise in medical nutrition, who contributed this dietary protocol advice to the participants. The office-based participants had an hour-long initial visit with Dr. Fuhrman, prior to starting the HND diet, and then had several 30- minute follow-up consultations. The web-based participants started the diet by reading the book Eat to Live and by accessing online support from the interactive website DrFuhrman.com, which provides web-based forums, recipes, and further information. Both the office-based participants and web-based participants had either visits or forum postings between 8/2007-8/2009. Data were collected and analyzed in 2009.
Ninety-seven charts and web postings from this twoyear period were reviewed for eligibility. Fifty-two of these met baseline HbA1C eligibility requirements. Of these, 27 were available for phone interviews. Thirteen of these met dietary compliance eligibility criteria and were included in the study. Of these, 7 were office-based and 6 were web-based. All the participants were required to have copies of lab results sent by clinical laboratories. Participants’ printed laboratory reports were collected from the charts for the office-based participants or were faxed/mailed by the participants or directly from the primary care physicians’ offices for the web-based participants. For the web-based participants, biometric indices (weight, height and blood pressure) were measured at their primary care physicians’ offices. For the officebased participants, the biometric parameters were measured at each visit by Dr. Fuhrman. Medication changes for the web-based participants were performed by their primary care physician. For the office-based participants, Dr. Fuhrman made the medication decisions.
This retrospective human subject analysis was reviewed and approved by the University of Pennsylvania’s Institutional Review Board.
Descriptive analysis was performed for the participant characteristics using mean and standard deviation (SD) for continuous variables and proportion for categorical variables. The change of parameters over time after the start of the HND diet was plotted for each individual participant. For statistical comparison, the mean parameters measured before and at the last time point after starting the HND diet were calculated and compared using both the absolute change and percentage change. p-values for testing whether absolute change and percentage change statistically differs from zero were determined using one-sample t-test. The correction for multiple statistical testing of many parameters was not considered as this study is a small pilot investigation.
p-value ≤ 0.05 was considered to be significant. For a participant with missing data in a parameter, the participant was excluded from the statistical comparison of this parameter, but was still included in the analysis of other parameters with complete data. The data analyses were performed using SAS v9.1 (SAS Institute Inc., Cary, NC).
3. RESULTS
Multiple baseline characteristics of each participant are illustrated (Table 1). The majority (69%) of participants was female. The demographics include 11 Caucasians and 2 African-Americans. The mean age was 57 years old, ranging from 30 to 80 years old. BMI at baseline ranged from 25 to 45.6 kg/m2. The mean HbA1C at baseline was 8.2%. Seventy-seven percent of the participants had hypertension (≥130/80 mmHg; based on ADA [23] and JNC7 [24] guidelines) and 92% had hyperlipidemia (based on the NCEP III guidelines [25]) before the HND diet. Sixty-two percent of participants had a family history of heart disease. The median length of follow-up with complete laboratory result time-points was 7 months (range: 5 to 42 months). During baseline and follow-up, a median of 3 laboratory result time-points (range: 2 to 8) per participant were obtained, with 92% having ≥3 laboratory result time-points. Most of the participants are/ were on the diet for much longer than the median follow-up period, though they did not have laboratory result time-points during this extended period.
After participants were on the HND diet for a median length of 7 months, most of the parameters showed significant improvements relative to pre-intervention levels (Table 2). The mean HbA1C dropped from 8.2% to
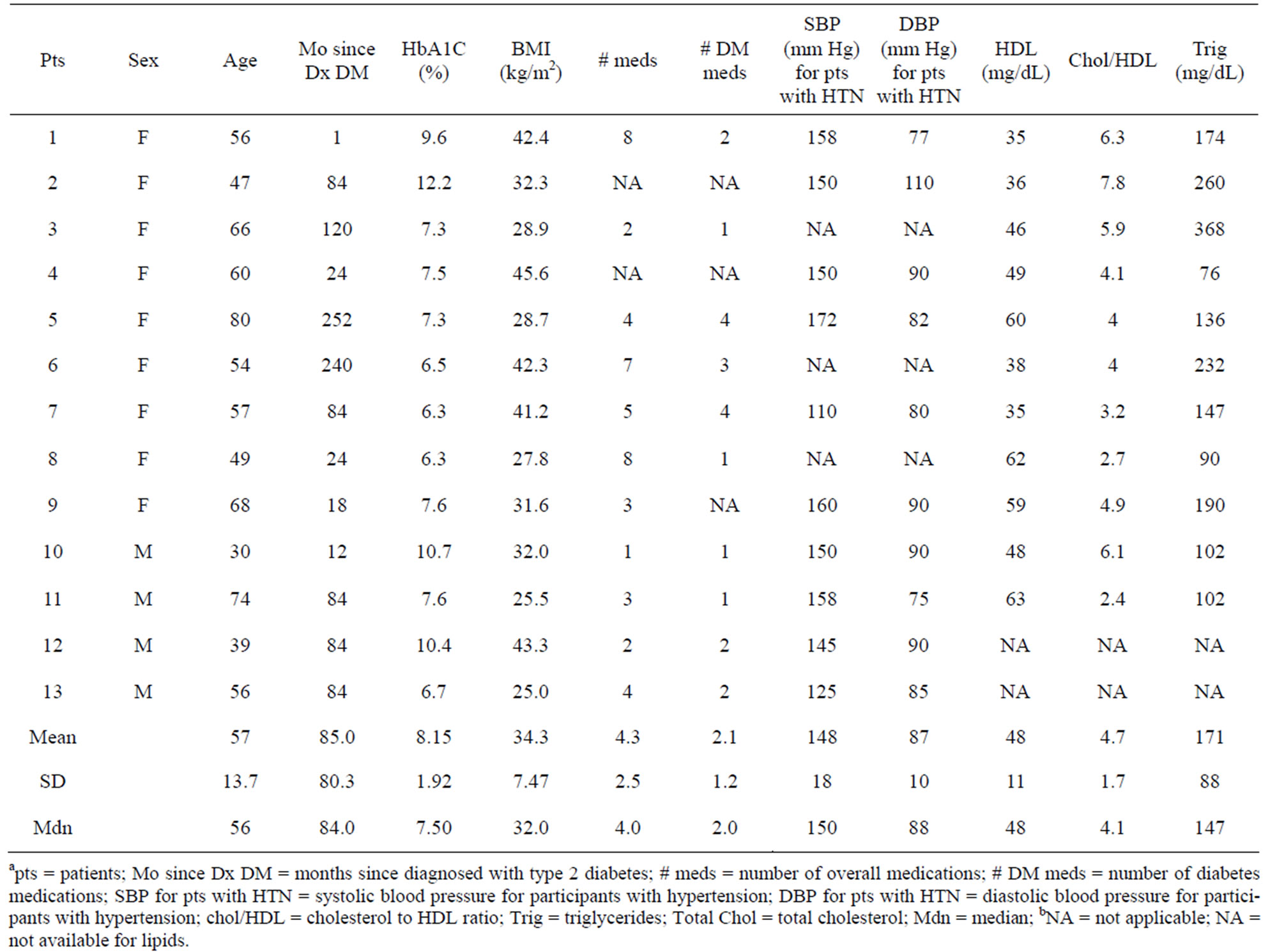
Table 1. Characteristics of participants at pre-intervention.
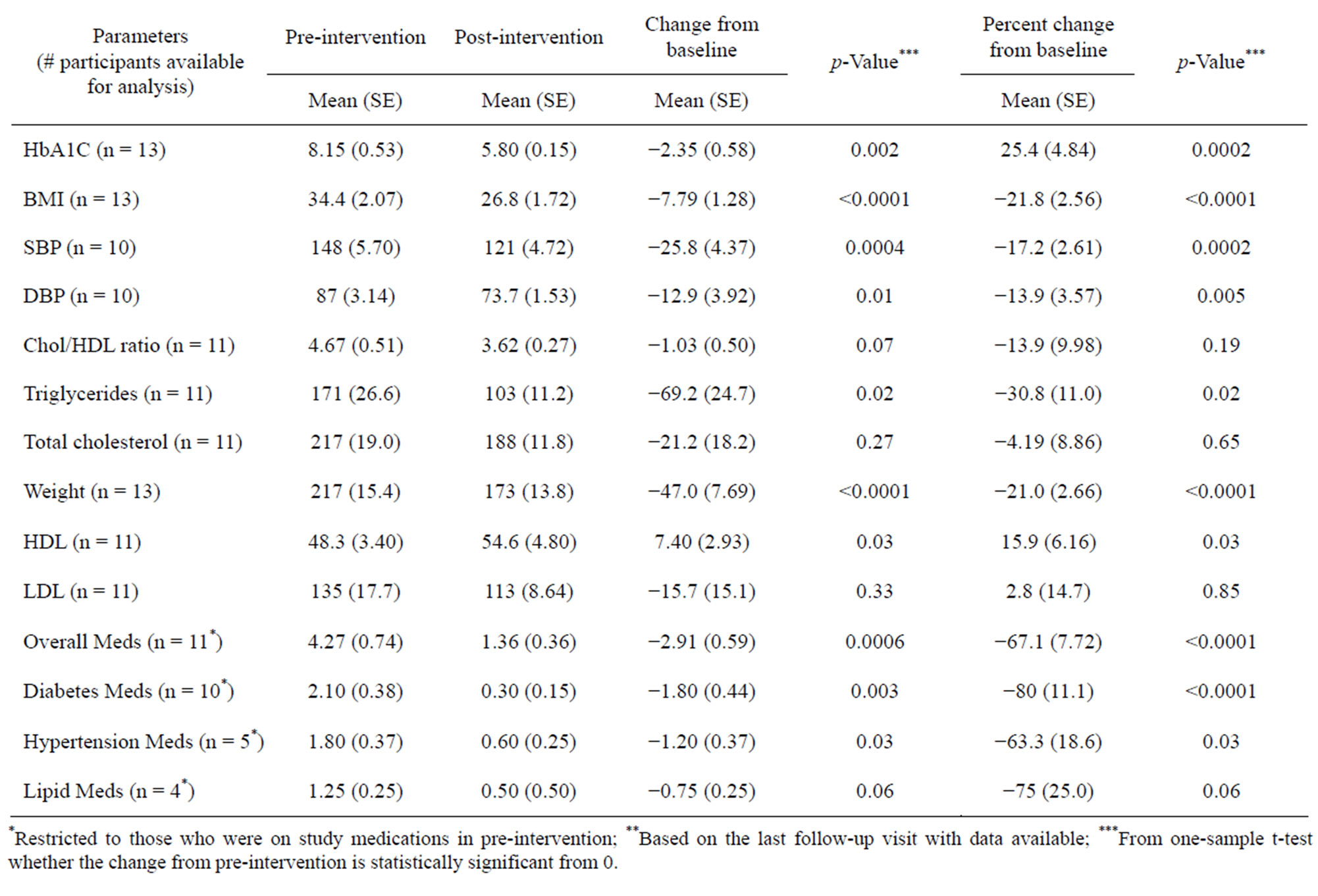
Table 2. A comparison of preand post-HND diet intervention parameters.
5.8%, a 30% relative reduction that reached statistical significance (p = 0.002). The mean systolic blood pressure (SBP) decreased from 148 mmHg to 121 mmHg, with an 18% reduction from the pre-intervention period (p = 0.0004). Triglycerides decreased significantly from the pre-intervention period, with an absolute reduction of 67.2 mg/dl (p = 0.02) from a mean of 170.6 mg/dl to 103.4 mg/dl. Cholesterol/HDL ratio changed from 4.67 to 3.62 (p = 0.07). Of the 11 participants on medications, the mean number of drugs dropped from 4.3 to 1.4 (p = 0.0006) and 90% of participants on diabetes medications (n = 10) were able to completely discontinue or reduce their diabetes drug therapy. The one participant (ID = 11) who did not change diabetes medication was on the lowest starting dose of Glucophage XR 500 mg, once daily.
All of the participants (n = 13) had a decrease in HbA1C (Figure 1(a)). Sixty-two percent reached nondiabetic HbA1C levels of <6.0% at the last data point for the HND diet. There was a substantial and rapid reduction in mean HbA1C at 1 - 4 months (n = 11) on the HND diet (8.15% at baseline, 6.52% at 1 - 4 months, p = 0.02), and it declined further, reaching normoglycemic levels, to a mean HbA1C 5.95% (p = 0.006 as compared to baseline) at 5 - 9 months (n = 11). Three participants (ID = 1, 4, 12) had HbA1C data substantially beyond this time period. One of the participants (ID = 1) started with glycosylated hemoglobin of 9.6%, reached a non-diabetic HbA1C of 5.4% at 15 months, and declined even further at 42 months to 4.8%, the participant’s last lab results. The second participant (ID = 4) initially had a HbA1C of 7.5%, which approached near normoglycemic levels of 6.2% at the last time point of 18 months. The HbA1C of the third participant (ID = 12) dropped substantially from 7.6% to a non-diabetic level of 5.9% at the last data point of 34 months.
Every participant (n = 13) showed a reduction in BMI (Figure 1(b)). Forty-six percent of participants reached a normal BMI (<25 kg/m2) [26] at their last data point. Five of the participants experienced an almost 10 kg/m2 or greater drop in BMI.
Each hypertensive type 2 diabetes participant (n = 10) in the study experienced a reduction in their SBP (Figure 1(c)) and 80% had a decrease of ≥20 mmHg. Of these participants, 7 experienced the considerable reduction within six months of starting the HND diet. Three hypertensive type 2 diabetes participants (ID = 1, 4, 13) had a sustained decline in their SBP with data out to 42, 18 and 34 months, respectively. Overall, participants who were on anti-hypertensive medications (n = 5) had a mean reduction in hypertensive medications of 67%.
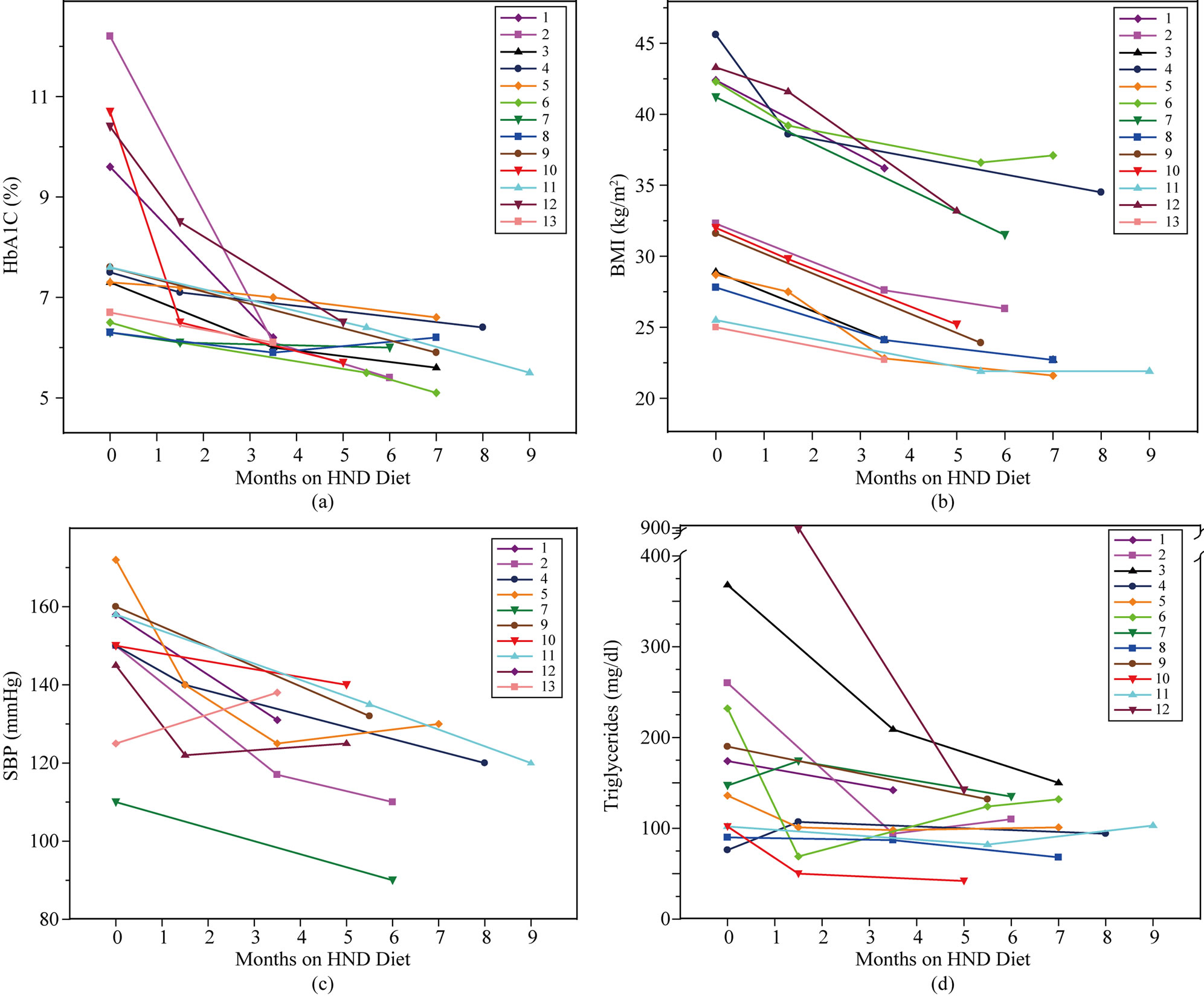
Figure 1. Graphs showing biometric data for each of the 13 study participants over time. Hemoglobin A1C (HbA1C; (a)), body mass index (BMI; (b)), systolic blood pressure (SBP; (c)) and triglycerides (d) are illustrated.
Most of the participants (n = 12) had a substantial drop in triglycerides within 6 months of starting the HND diet (Figure 1(d)). There were 11 participants who started the HND diet with dyslipidemia. Three participants (ID = 2, 3, 6) had triglycerides at pre-intervention that were >200 mg/dl. Their mean at baseline was 286 mg/dl with a precipitous mean reduction of 161 points. Another participant (ID = 12), whose pre-intervention levels were not obtained, had triglycerides of 885 mg/dl at 2 months which dropped to 142 mg/dl in 3 months, while still severely obese.
4. DISCUSSION
This case series of 13 type 2 diabetes participants demonstrated the mean reduction of 2.35% in the HbA1C, achieving statistical significance, with 62% reaching non-diabetic levels (HbA1C < 6.0%). Those with hypertension reached a mean normotensive level.
Triglycerides were reduced while HDL was increased, both significantly. There was a substantial drop in the total number of drugs, and 90% of participants discontinued or decreased diabetes medications. None of these effects was due to hypoglycemic, hypertensive or lipid lowering medications, since there was no added or increased dose of medication in the study participants.
In addition to the substantial decrease in diabetes medications (n = 10) seen in this HND diet case series, 85% of participants (n = 11) were able to discontinue or reduce the dose of overall medications, including diabetes, hypertensive and lipid therapies. These participants were not nascent to diabetes, but had a mean disease duration of 7.1 years prior to the HND diet. The overall number of medications was reduced by 67%.
Although there are no head-to-head comparisons with other plant-based diets in type 2 diabetes patients, this study provides an initial assessment of the HND diet’s effectiveness for diabetes. In several studies, it has been demonstrated that with a low fat vegan diet, type 2 diabetes could be better controlled than with the ADA diet [27,28]. In a randomized clinical trial contrasting a low fat vegan diet with the ADA diet, 43% of patients in the low fat vegan arm and 26% of patients in the ADA arm had a reduction in diabetes medications. Also, the HbA1C was reduced by a mean of 0.96% in 49 patients in the low fat vegan arm, and in the ADA arm, HbA1C was reduced by a mean of 0.56% in 50 patients [27].
Since there was a selection bias introduced into our study based on participant choice to participate on Dr. Fuhrman’s website and/or office visits with Dr. Fuhrman, an accurate comparison of diet programs would best be accomplished by head-to-head analysis in a randomized prospective trial. However, the magnitude of effect of the HND diet in this case series indicates that the HND diet can be very effective in some participants.
Prior to starting the HND diet, 9 participants experienced diabetes-related complications and/or symptoms, including peripheral neuropathy in five participants, hypoglycemic episodes, cerebrovascular attack (CVA) or transient ischemic attack (TIA), and lethargy. All of the participants reported a reduction or complete dissipation of these symptoms. Further, none of the participants during the study period had any heart attacks, strokes, limb amputations, nephropathy or visual complaints.
Atherogenic dyslipidemia is a risk factor for heart disease [29]. Four of the participants in the HND diet case series (ID = 2, 3, 6, 12) had atherogenic dyslipidemia, which are triglycerides > 200 mg/dl and HDL < 50 mg/dl for a female or < 40 mg/dl for a male. After commencing the HND diet, all four of the participants’ triglyceride levels were substantially reduced by over 100 points to 150 mg/dl or lower. The mean HDL rose 6.3 points to 54.6 mg/dl (n = 11), a statistically significant result compared to pre-HND diet levels (p = 0.03).
The CDC reports that 75% of type 2 diabetes patients also have hypertension, [30] of which only 36% are controlled [2]. The risk for cardiovascular disease begins to increase when blood pressure is > 115/75 mmHg. For every 20 mmHg increase in SBP and/or 10 mmHg increase in diastolic blood pressure, the risk for heart disease doubles [24]. Hypertensive type 2 diabetes participants (n = 10) in this HND diet case series showed a significant reduction in mean blood pressure from 148/87 mmHg to a normotensive level of 121/74 mmHg.
One potential mechanism for the decreases in glycemic levels, hypertension and hyperlipidemia in this HND diet case series is weight loss. However, the mean BMI at the last intervention point was 26.6 kg/m2, which is considered overweight, yet the mean HbA1C reached a normoglycemic level of 5.8%. Six of the participants whose BMIs decreased were still overweight or obese, yet 3 out of 6 reached normoglycemic levels (ID = 2, 6, 10). The remaining three (ID = 4, 7, 12) reached near normoglycemic levels. In fact, two of the participants (ID = 4 and 6) had BMIs at their last data points that are considered severely obese. Even participants (ID = 4, 6, 11, 13) who experienced either an increase or no change in BMIs (mean= +1.3%) between two data points demonstrated a decrease in HbA1C (mean= −6.8%) between these data points. Thus, the HND diet’s beneficial effects on HbA1C appear to extend beyond weight loss.
In this case series, the HND diet has shown substantial sustainability and feasibility. The mean duration of the study with the 13 participants was 12.3 months. Of these participants, 62% were still on the diet at the study end point. Though five participants’ HND diet compliance eventually lapsed, they all expressed interest in resuming, and four have been back on the diet for at least a few months. In terms of feasibility, 46% of the participants were able to garner knowledge about the HND diet for type 2 diabetes from the book Eat to Live and/or the supplemental interactive website. These six participants did not have a phone consult or office visit before initiating the diet or during the study period, though they could ask questions on the “ask the doctor” portion of the website. This is an indicator of the accessibility and clarity of the HND diet.
However, there are limitations to this study. It was a small retrospective case series with no control group. The study had selection bias, primarily because participants who reported their results on the website or chose to see Dr. Fuhrman in person may not represent the “typical” type 2 diabetes patient. In the study, 9 of 13 participants were female; its effectiveness in males needs further study. The HND diet may not work as effectively for everyone. For some, the HND diet may fail for numerous reasons, especially compliance. In this case series, participants were required to be at least 90% compliant with the diet. Of course, many patients also are not compliant with medications. To be successful, patients must have the proper knowledge and willpower. For the highly motivated individual, the HND diet appears to be an important weapon in the arsenal against type 2 diabetes.
5. CONCLUSION
This HND diet case series suggests benefits for patients with type 2 diabetes, its complications, and with some co-morbidities, such as heart disease risk, hypertension and hyperlipidemia. The HND diet may work well for some type 2 diabetes patients, but compliance with the HND diet may be an issue. This small case series has selection bias. However, the results of this study demonstrate that the HND diet can be a very effective intervention for some with type 2 diabetes. Therefore, further study is needed
REFERENCES
- Nathan, D.M., Buse, J.B., Davidson, M.B., Ferrannini, E., Holman, R.R., Sherwin, R. and Zinman, B. (2008) American diabetes association; European association for study of diabetes medical management of hyperglycemia in type 2 diabetes: A consensus algorithm for the initiation and adjustment of therapy. Diabetes Care, 32, 193-203. doi:10.2337/dc08-9025
- Saydah, S.H., Fradkin, J. and Cowie, C. (2004) Poor control of risk factors for vascular disease among adults with previously diagnosed diabetes. Journal of the American Medical Association, 291, 335-342. doi:10.1001/jama.291.3.335
- Montonen, J., Knekt, P., Härkänen, T., Järvinen, R., Heliövaara, M., Aromaa, A. and Reunanen, A. (2005) Dietary patterns and the incidence of type 2 diabetics. American Journal of Epidemiology, 161, 219-227. doi:10.1093/aje/kwi039
- Ylönen, K., Alfthan, G., Groop, L., Saloranta, C., Aro, A., Virtanen, S.M. and the Botnia Research Group (2003) Dietary intakes and plasma concentrations of carotenoids and tocopherols in relation to glucose metabolism in subjects at high risk of type 2 diabetes: the Botnia dietary study. American Journal of Clinical Nutrition, 77, 1434- 1441.
- Jenkins, D.J., Kendall, C.W., Marchie, A., Jenkins, A.L., Augustin, L.S., Ludwig, D.S., Barnard, N.D. and Anderson J.W. (2003) Type 2 diabetes and the vegetarian diet. American Journal of Clinical Nutrition, 78, 610S-616S.
- Bourn, D.M., Mann, J.I., McSkimming, B.J., Waldron, M.A. and Wishart, J.D. (1994) Impaired glucose tolerance and NIDDM: does lifestyle intervention program have an effect? Diabetes Care, 17, 1311-1319. doi:10.2337/diacare.17.11.1311
- Ford, E.S. and Mokdad, A.H. (2001) Fruit and vegetable consumption and diabetes mellitus incidence among US adults. Preventive Medicine, 32, 33-39. doi:10.2337/diacare.17.11.1311
- Giammarioli, S., Filesi, C., Vitale, B., Cantagallo, A., Dragoni, F. and Sanzini, E. (2004) Effect of high intakes of fruit and vegetables on redox status in type 2 onset diabetes: a pilot study. International Journal for Vitamin and Nutrition Research, 74, 313-320. doi:10.1024/0300-9831.74.5.313
- Vasdev, S., Gill, V. and Singal P. (2007) Role of advanced glycation end products in hypertension and atherosclerosis: therapeutic implications. Cell Biochemistry and Biophysics, 49, 48-63. doi:10.1007/s12013-007-0039-0
- Cervantes-Laurean, D., Schramm, D.D., Jacobson, E.L., Halaweish, I., Bruckner, G.G. and Boissonneault, G.A. (2006) Inhibition of advanced glycation end product formation on collagen by rutin and its metabolites. Journal of Nutritional Biochemistry, 17, 531-540. doi:10.1016/j.jnutbio.2005.10.002
- Benetou, V., Trichopoulou, A., Orfanos, P., Naska, A., Lagiou, P., Boffetta, P. and Trichopoulos, D. (2008) Conformity to traditional Mediterranean diet and cancer incidence: the Greek EPIC cohort. British Journal of Cancer, 99, 191-195. doi:10.1038/sj.bjc.6604418
- Martinez-Gonzalez, M.A., de la Fuente-arrillaga, C., Nunez-Cordoba, J.M., Basterra-Gortari, F.J., Beunza, J.J., Vazquez, Z., Benito, S., Tortosa, A. and Bes-Rastrollo, M. (2008) Adherence to Mediterranean diet and risk of developing diabetes: prospective cohort study. British Medical Journal, 336, 1348-1351. doi:10.1136/bmj.39561.501007.BE
- Trichopoulou, A. and Lagiou, P. (2007) Healthy traditional Mediterranean diet: an expression of culture, history, and lifestyle. Nutrition Reviews, 55, 383-389. doi:10.1111/j.1753-4887.1997.tb01578.x
- Lanou, A.J. (2009) Should dairy be recommended as part of a healthy vegetarian diet? Counterpoint. American Journal of Clinical Nutrition, 89, 1638S-1642S. doi:10.3945/ajcn.2009.26736P
- Kris-Etherton, P.M., Hu, F.B., Ros, E. and Sabaté J. (2008) The role of tree nuts and peanuts in prevention of coronary heart disease. Journal of Nutrition, 138, 1746S- 1751S.
- Jiang, R., Manson, J.E., Sampfer, M.J., Liu, S., Willett, W.C. and Hu, F.B. (2002) Nut and peanut butter consumption and risk of type 2 diabetes in women. Journal of the American Medical Association, 288, 2554-2560. doi:10.1001/jama.288.20.2554
- Sabate, J. and Ang, Y. (2009) Nuts and health outcomes: new epidemiologic evidence. American Journal of Clinical Nutrition, 89, 1643S-1648S. doi:10.3945/ajcn.2009.26736Q
- Albert, C.M., Gaziano, J.M., Willett, W.C. and Manson, J.E. (2002) Nut consumption and decreased risk of sudden cardiac death in the Physicians’ Health Study. Archives of Internal Medicine, 162, 1382-1387.
- Hu, F.B., Stampfer, M.J., Manson, J.E., Rimm, E.B., Colditz, G.A., Rosner, B.A., Speizer, F.E., Hennekens, C.H. and Willett, W.C. (1998) Frequent nut consumption and risk of coronary heart disease in women: prospective cohort study. British Medical Journal, 317, 1341-1345. doi:10.1136/bmj.317.7169.1341
- Sabate, J. (1999) Nut consumption, vegetarian diets, ischemic heart disease risk and all-cause mortality: evidence from epidemiologic studies. American Journal of Clinical Nutrition, 70, 500S-503S.
- Li, T.Y., Brennan, A.M., Weddick, N.M., Mantzoros, C., Rifai, N. and Hu, F.B. (2009) Regular consumption of nuts is associated with a lower risk of cardiovascular disease in women with Type 2 Diabetes. Journal of Nutrition, 139, 1333-1338. doi:10.3945/jn.108.103622
- Jenkins, D.J., Hu, F.B., Tapsell, L.C., Josse, A.R. and Kendall, C.W. (2008) Possible benefit of nuts in Type 2 diabetes. Journal of Nutrition, 138, 1752S-1756S.
- American Diabetes Association (2008) Standards of medical care in diabetes-2008. Diabetes Care, 31, S12-S54. doi:10.2337/dc08-S012
- Chobanian, A., Bakris, G., Black, H.R., Cushman, W.C., Green, L.A., Izzo, J.L. Jr., Jones, D.W., Materson, B.J., Oparil, S, Wright, J.T., Jr., Roccella, E.J. and the National High Blood Pressure Education Program Coordinating Committee (2003) The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Journal of the American Medical Association, 289, 2560-2571. doi:10.1001/jama.289.19.2560
- Expert Panel on Detection, Evaluation and Treatment of High Blood Cholesterol in Adults (2001) Executive summary of the third report of the national cholesterol education program (ncep) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (adult treatment panel III). Journal of the American Medical Association, 285, 2486-2497. doi:10.1001/jama.285.19.2486
- Classification of overweight and obesity by BMI, waist circumference, and associated diseaserRisks (2009) http://www.nhlbi.nih.gov/health/public/heart/obesity/lose_wt/bmi_dis.htm
- Barnard, N.D., Cohen, J., Jenkins, D.J., Turner-McGrievy, G., Gloede, L., Jaster, B., Seidl, K., Green, A.A. and Talpers, S. (2006) A low-fat vegan diet improves glycemic control and cardiovascular risk factors in a randomized clinical trial in individuals with type 2 diabetes. Diabetes Care, 29, 1777-1783. doi:10.2337/dc06-0606
- Barnard, N.D., Cohen, J., Jenkins, D.J., Turner-McGrievy, G., Gloede, L., Green, A. and Ferdowsian, H. (2009) A low-fat vegan diet and a conventional diabetes diet in the treatment of type 2 diabetes: a randomized, controlled, 74-wk clinical trial. American Journal of Clinical Nutrition, 89, 1588S-1596S. doi:10.3945/ajcn.2009.26736H
- Arca, M., Montali, A., Valiante, S., Campagna, F., Pigna, G., Paoletti, V., Antonini, R., Barilla, F., Tanilli, G., Vestri, A. and Gaudio, C. (2007) Usefulness of atherogenic dyslipidemia for predicting cardiovascular risk in patients with angiographically defined coronary artery disease. American Journal of Cardiology, 100, 1511-1516. doi:10.1016/j.amjcard.2007.06.049
- National Diabetes Statistics 2007 (2008) Internet accessed 25 May 2009. http://www.diabetes.niddk.nih.gov/DM/PUBS/statistics/

