Advances in Biological Chemistry
Vol. 2 No. 4 (2012) , Article ID: 24817 , 5 pages DOI:10.4236/abc.2012.24051
Inhibitors of protein kinases affecting cAMP-dependent proteolysis of GATA-6
![]()
Department of Molecular Biology, School of Pharmacy, Iwate Medical University, Shiwa-Gun, Japan
Email: *mmaeda@iwate-med.ac.jp
Received 25 August 2012; revised 28 September 2012; accepted 3 October 2012
Keywords: cAMP; GATA-6; Kinase Inhibitor; Proteolysis; Transcription Factor
ABSTRACT
We screened 95 kinase inhibitors whether they affect cAMP-dependent proteolysis of GATA-6 or not. Among them 7 inhibitors inhibited the proteolysis at the concentration range of μM around their IC50. They are inhibitors for protein kinase A (H-89 and 4- cyano-3-methylisoquinoline), c-Jun N-terminal kinase (SP600125), phosphatidylinositol 3-kinase (Wortmannin and LY-294002), casein kinase II (TBB) and cyclin dependent kinase (Cdk1/2 inhibitor III). It is of interest how these kinases play roles in the degradation process of GATA-6 since this transcription factor is essential for development and tissue-specific gene expression of mammals. Inhibitors identified in this study would be helpful to study molecular mechanisms of phenomena in which GATA-6 participates.
1. INTRODUCTION
The sequence-specific DNA-binding protein GATA-6 [1,2] is essential for early developmental processes and tissue-specific gene expression of mammals [3]. GATA- 6 stably expressed in CHO-K1 cells is specifically degraded by proteasomes upon elevation of cAMP concentration [4]. It is suggested by inhibitor studies that this signaling pathway is mediated by protein kinase A (A-kinase) but not by other kinases such as C-kinase, MAP-kinase (MEK and p38), CAM kinase II, and Src kinase [5].
When GATA-6 was fixed on the cytoplasmic side of endoplasmic reticulum membrane as a fusion protein with the carboxy-terminal membrane domain of SREBP, it was degraded similarly to that expressed in the nucleus, suggesting that the degradation of GATA-6 occurs at least in the cytoplasm [6]. A similar degradation pathway is demonstrated for proteolysis of Sp1 in NRK cells [7,8]. Although the phenomenon of cAMP-dependent proteolysis is evident in mammalian cultured cells, its physiological role has not been unveiled.
We could obtain mutant clones in which GATA-6 is not degraded even in the presence of dbcAMP although mutation sites were unidentified [9]. It is also demonstrated that two signaling pathways branch off at the point of A-kinase [10]. However, none is known for component molecules participated in the signaling pathway of GATA-6 degradation except cAMP and Akinase.
In this study, we compared 95 kinase inhibitors [11] since we are expecting that there are multiple cross-talks between A-kinase and different kinases hitherto not being examined by us. Actually we identified 5 kinase inhibitors that prohibit protein kinases other than Akinase. These kinase inhibitors will give us novel insights into how kinase cascade operates in the proteolysis of GATA-6.
2. MATERIALS AND METHODS
2.1. Materials
The SCADS inhibitor kit III was provided from Screening Committee of Anticancer Drugs supported by Grantin-Aid for Scientific Research on Priority Area “Cancer” from The Ministry of Education, Culture, Sports, Science and Technology, Japan [11]. Cyclin-dependent kinase (Cdk)1/2 inhibitor III, 4,5,6,7-tetrabromobenzotriazole (TBB), Wortmannin, and 4-cyano-3-methylisoquinoline were obtained from Calbiochem. LY-294002 and SP600125 from Wako Chemicals and Alexis Biochemicals, respectively.
2.2. Cell Culture
CHO-K1 cells (1.5 × 106 per well, in a 100 mm dish) stably expressing rat GATA-6 (tc1-17a cells) [8] were cultured in Ham F-12 medium (Sigma) containing 7% (v/v) fetal bovine serum (FBS) (Gibco BRL) at 37˚C for 24 h, and then the culture medium was replaced by the fresh one containing both 2 mM dbcAMP (Sigma) and 1 µM each of kinase inhibitors.
2.3. Immunoblotting Analysis of GATA-6
Cells treated with both dbcAMP and a kinase inhibitor for 24 h were washed with phosphate-buffered saline [10 mM sodium phosphate buffer (pH 7.2), 137 mM NaCl, 3 mM KCl] (PBS), and scraped into 1 ml PBS with rubber policeman. Cells were precipitated in micro-centrifuge (3000 rpm) for 2 min at 4˚C, and then suspended in 200 µL Buffer A [10 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES)-KOH (pH 7.6), 10 mM KCl, 1.5 mM MgCl2, 0.5 mM dithiothreitol (DTT), 0.2 mM phenylmethanesulfonyl fluoride (PMSF)]. After incubation for 10 min at 4˚C, the suspension was mixed with vortex mixer, and centrifuged (3000 rpm) for 10 min at 4˚C. The nuclear pellet was suspended for 30 min at 4˚C in 50 µl Buffer C [20 mM HEPES-KOH (pH 7.6), 400 mM NaCl, 1.5 mM MgCl2, 0.2 mM ethylenediaminetetraacetic acid (EDTA), 0.5 mM DTT, 0.2 mM PMSF, 25% (v/v) glycerol]. The suspension was centrifuged (15,000 rpm) for 2 min at 4˚C, and the supernatant was stored at –80˚C until use [8]. Protein concentrations were determined with a BCA Protein Assay (Pierce) using bovine serum albumin (Fraction V, Sigma) as a standard [12].
Proteins (10 µg) were subjected to sodium dodecylsulfate (SDS)-polyacrylamide gel-electrophoresis [7.5% (w/v) separation gel] [13], and then electro-blotted on ImmobilonTM-P membrane (Millipore) [14]. The GATA- 6 was detected with AmershamTM ECL Western blotting analysis system [×2000 and ×5000 dilution for antiGATA-6 antibodies [4] and horseradish peroxidase (HRP)-linked donkey anti-rabbit immunoglobulin (Ig) (Amersham Biosciences), respectively].
3. RESULTS
3.1. Effects of Kinase Inhibitors on cAMP-Dependent Proteolysis of GATA-6
We have previously reported that the activation of Akinase by cAMP caused the degradation of GATA-6 by proteasomes [4]. However, component molecules communicating between A-kinase and proteasomes have not been identified. To address the cross-talk between Akinase and other protein kinases [4,5], we examined the effects of kinase inhibitors (SCADS inhibitor kit III) [11] on cAMP-dependent proteolysis of GATA-6 using tc1- 17a cells that is a CHO-K1 clone stably expressing GATA-6.
Among 95 kinase inhibitors [11], H-89 [15] (A-kinase inhibitor) exhibited an inhibitory effect as we previously reported, and another A-kinase inhibitor 4-cyano-3- methylisoquinoline [16] also inhibited the proteolysis. These results confirmed that A-kinase functions in the proteolysis of GATA-6. Interestingly other five inhibitors (Cdk1/2 inhibitor III [17], TBB [18], SP600125 [19], LY-294002 [20], and Wortmannin [21]) for serinethreonine kinases such as cyclin dependent kinase (CDK)1 [22], CDK2 [23], casein kinase II (CKII) [24], c-Jun N-terminal kinase (JNK) [25], and phosphatidylinositol 3-kinase (PI3K) [26], respectively, strongly inhibited GATA-6 degradation (Figure 1).
On the other hand, inhibitors for tyrosine kinases did not have any effects. Only weak effects could be detected for several inhibitors (sample No. 28, 29, 30, 43 and 65 shown in Figure 1). However, we focused on the 6 inhibitors that showed strong inhibitory effects in the following experiments.
3.2. Effective Concentrations of Kinase Inhibitors against cAMP-Dependent Proteolysis of GATA-6
Next we examined the effective concentration of 6 kinase inhibitors (Cdk1/2 inhibitor III, TBB, SP600125, LY-294002, Wortmannin, and 4-cyano-3-methylisoquinoline) on the inhibition of GATA-6 degradation. As shown in Figure 2., all inhibitors showed inhibition at the concentration of 1.0 µM similar to Figure 1. SP600125 showed strong inhibition since they were effective at the concentration as low as 0.01 µM. Wortmannin, and 4-cyano-3-methylisoquinoline were also inhibitory at relatively low concentration of 0.05 µM. However, Cdk1/2 inhibitor III and TBB were effective at 0.5 and 1.0 µM, respectively. LY-294002 was weak inhibitory at 1.0 µM. These results suggest that A-kinase together with other kinases such as JNK, PI3K, CKII and CDK could be components of signal-induced proteolysis of GATA-6.
4. DISCUSSION
We screened 95 kinase inhibitors whether they could inhibit cAMP-dependent proteolysis of GATA-6, and found that 7 of them strongly and moderately inhibited the induced proteolysis. Two (H-89 and 4-cyano-3- methylisoquinoline) are inhibitors of A-kinase, indicating that our previous observation [4] is reproducible since H-89 is again positive in the present study. Furthermore, KN93, PD98059, SB239063, and PP1 also showed negative effects [5].
Other five effective inhibitors are those of JNK (SP600125), PI3K (Wortmannin and LY-294002), CKII (TBB) and CDK (Cdk1/2 inhibitor III). Titration experiments of these inhibitors demonstrated that they inhibited the proteolysis at the concentration around their IC50 [27]. Thus their potential targets could function in
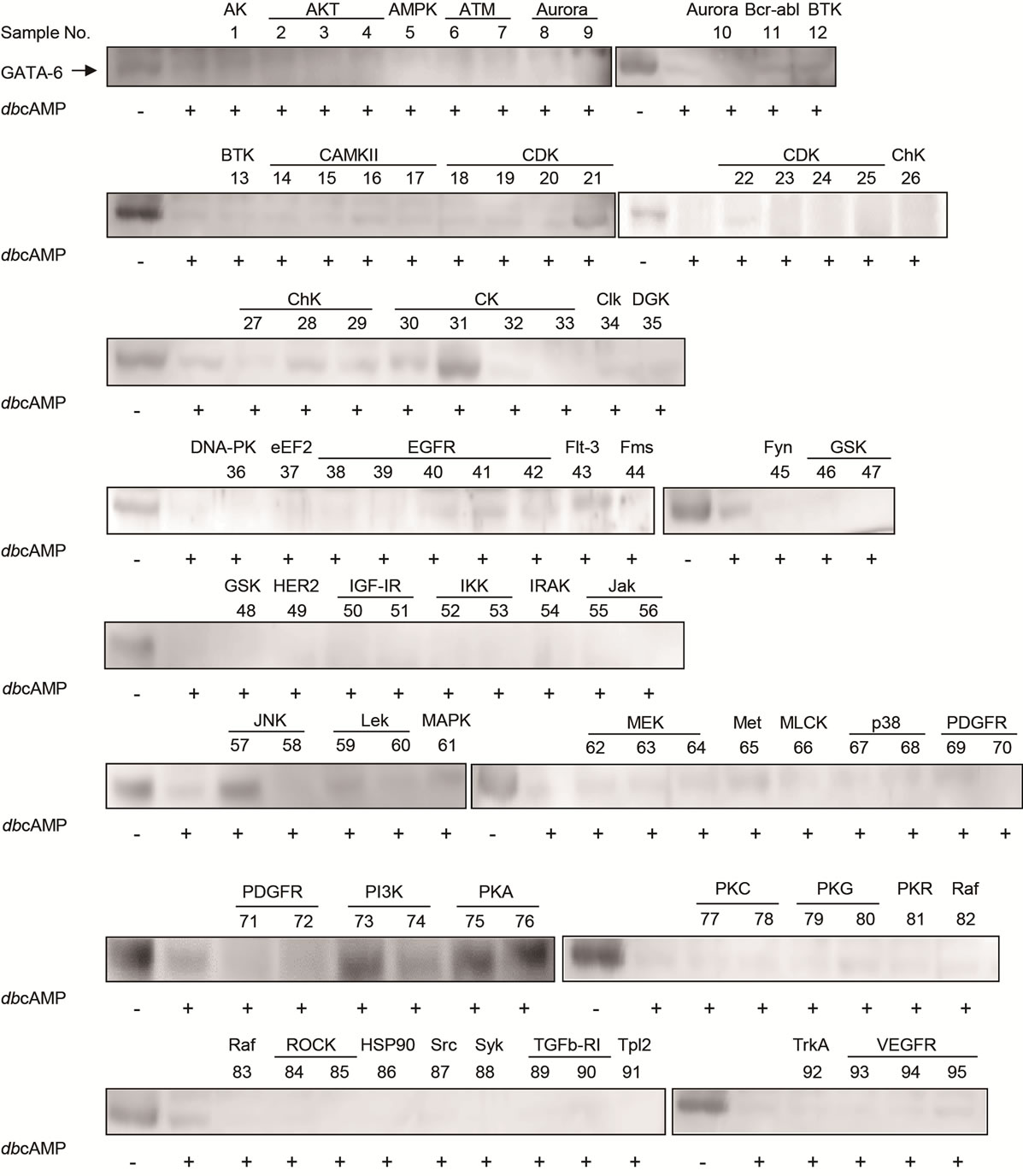
Figure 1. cAMP-dependent proteolysis of GATA-6. The tc1-17a cells expressing GATA-6 were treated with 2 mM dbcAMP and 1 µM of each kinase inhibitors for 24 h. Nuclear extract was prepared as described in Materials and Methods. GATA-6 was detected by western blotting with anti-GATA-6 antibodies [4]. Compounds exhibiting potent inhibition for GATA-6 degradation are sample No. 21, 31, 57, 73, 74, 75, 76. HSP90 is not a protein kinase, but known as a modulator which complexes with many protein kinases [36].
the GATA-6 degradation pathway. We further demonstrated that JNK not only induces proteolysis of GATA-6 by proteasomes but also stimulates export of GATA-6 from nucleus to cytoplasm [28]
GATA-6 is essential for developmental process of endoderm [29], and specific gene expression in differentiated cells [30,31]. However, little is known on signaling pathways governing complex regulation of gene expression. The inhibitors identified in the present study and the activators of their potential target kinases would give chances to clarify signal dependent proteolysis of GATA-6 and its physiological role(s) in cell type specific gene regulation.
The positive effects of inhibitors for JNK, PI3K, CKII, and CDK suggest that these protein kinases could be functionally interacted. These kinases are known to be essential for stress responses such as ultraviolet, heat, and hypotonicity, cell cycle regulation, differentiation, growth and so on [22-26]. It is reported that JNK directly phosphorylates some transcription factors, and regulates

Figure 2. Effective concentration of kinase inhibitors on cAMP-dependent proteolysis of GATA-6. The tc1-17a cells were treated with 2 mM dbcAMP and each concentration of kinase inhibitors for 24 h. GATA-6 was detected as described in the legend to Figure 1.
stability of the target transcription factors through ubiquitin-proteasome system [32,33]. Furthermore, DNA binding activities of Jun and c-Myc transcription factors are controlled via phosphorylation process by CKII [34, 35]. Thus it would be of interest how these kinases communicate with the signaling pathway for cAMPdependent proteolysis. These lines of experiments are now in progress and the results will be reported in near future.
5. ACKNOWLEDGEMENTS
We thank the Screening Committee of Anticancer Drugs supported by a Grant-in-Aid for Scientific Research on Priority Area “Cancer” from MEXT for the SCADS inhibitor kit III. This research was supported in part by grants from MEXT for Strategic Medical Science Research Centers, 2010-2014 (The MIAST Project) and JSPS KAKENHI Grant Number 24701032 to H.U.
![]()
![]()
REFERENCES
- Tamura, S., Wang, X.H., Maeda, M., et al. (1993) Gastric DNA-binding proteins recognize upstream sequence motifs of parietal cell-specific genes. Proceedings of the National Academy of Sciences, 90, 10876-10880. doi:10.1073/pnas.90.22.10876
- Maeda, M., Kubo, K., Nishi, T., et al. (1996) Roles of gastric GATA DNA-binding proteins. The Journal of Experimental Biology, 199, 513-520.
- Maeda, M., Ohashi, K. and Ohashi-Kobayashi, A. (2005) Further extension of mammalian GATA-6. Development, Growth & Differentiation, 47, 591-600. doi:10.1111/j.1440-169X.2005.00837.x
- Nakagawa, R., Sato, R., Futai, M., et al. (1997) Gastric GATA-6 DNA-binding protein: Proteolysis induced by cAMP. FEBS Letters, 408, 301-305. doi:10.1016/S0014-5793(97)00443-2
- Ishida, A., Iijima, R., Kobayashi, A., et al. (2005) Characterization of cAMP-dependent proteolysis of GATA-6. Biochemical and Biophysical Research Communications, 332, 976-981. doi:10.1016/j.bbrc.2005.05.042
- Tsuge, T., Uetani, K., Sato, R., et al. (2008) Cyclic AMP-dependent proteolysis of GATA-6 expressed on the intracellular membrane. Cell Biology International, 32, 298-303. doi:10.1016/j.cellbi.2007.10.005
- Han, I. and Kudlow, J.E. (1997) Reduced O glycosylation of Sp1 is associated with increased proteasome susceptibility. Molecular and Cellular Biology, 17, 2550-2558.
- Su, K., Roos, M.D., Yang, X., et al. (1999) An N-terminal region of Sp1 targets its proteasome-dependent degradation in vitro. The Journal of Biological Chemistry, 274, 15194-15202. doi:10.1074/jbc.274.21.15194
- Maeda, M., Ishida, A., Ni, L., et al. (2005) Isolation of CHO-K1 clones defective in cAMP-dependent proteolysis, as determined by the stability of exogenously expressed GATA-6. Biochemical and Biophysical Research Communications, 329, 140-146. doi:10.1016/j.bbrc.2005.01.118
- Robinson-White, A. and Stratakis, C.A. (2002) Protein kinase A signaling: “Cross-talk” with other pathways in endocrine cells. Annals of the New York Academy of Sciences, 968, 256-270. doi:10.1111/j.1749-6632.2002.tb04340.x
- SCADS inhibitor kit (2012). http://gantoku-shien.jfcr.or.jp/kit.html
- Bradford, M.M. (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry, 72, 248-254. doi:10.1016/0003-2697(76)90527-3
- Laemmli, U.K. (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature, 227, 680-685. doi:10.1038/227680a0
- Towbin, H., Staehelin, T. and Gordon, J. (1979) Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: Procedure and some applications. Proceedings of the National Academy of Sciences, 76, 4350-4354. doi:10.1073/pnas.76.9.4350
- Chijiwa, T., Mishima, A., Hagiwara, M., et al. (1990) Inhibition of forskolin-induced neurite outgrowth and protein phosphorylation by a newly synthesized selective inhibitor of cyclic AMP-dependent protein kinase, N-[2- (p-bromocinnamylamino)ethyl]-5-isoquinolinesulfonam de (H-89), of PC12D pheochromocytoma cells. The Journal of Biological Chemistry, 265, 5267-5272.
- Lu, Z.X., Quazi, N.H., Deady, L.W., et al. (1996) Selective inhibition of cyclic AMP-dependent protein kinase by isoquinoline derivatives. Biological chemistry HoppeSeyler, 377, 373-384. doi:10.1515/bchm3.1996.377.6.373
- Lin, R., Connolly, P.J., Huang, S., et al. (2005) 1-Acyl- 1H-[1,2,4]triazole-3,5-diamine analogues as novel and potent anticancer cyclin-dependent kinase inhibitors: Synthesis and evaluation of biological activities. Journal of Medicinal Chemistry, 48, 4208-4211. doi:10.1021/jm050267e
- Szyszka, R., Grankowski, N., Felczak, K., et al. (1995) Halogenated benzimidazoles and benzotriazoles as selective inhibitors of protein kinases CK I and CK II from Saccharomyces cerevisiae and other sources. Biochemical and Biophysical Research Communications, 208, 418- 424. doi:10.1006/bbrc.1995.1354
- Bennett, B.L., Sasaki, D.T., Murray, B.W., et al. (2001) SP600125, an anthrapyrazolone inhibitor of Jun N-terminal kinase. Proceedings of the National Academy of Sciences, 98, 13681-13686. doi:10.1073/pnas.251194298
- Vlahos, C.J., Matter, W.F., Hui, K.Y., et al. (1994) A specific inhibitor of phosphatidylinositol 3-kinase, 2-(4- morpholinyl)-8-phenyl-4H-1-benzopyran-4-one (LY294002). The Journal of Biological Chemistry, 269, 5241-5248.
- Powis, G., Bonjouklian, R., Berggren, M.M., et al. (1994) Wortmannin, a potent and selective inhibitor of phosphatidylinositol-3-kinase. Cancer Research, 54, 2419- 2423.
- Lee, M.G. and Nurse, P. (1987) Complementation used to clone a human homologue of the fission yeast cell cycle control gene cdc2. Nature, 327, 31-35. doi:10.1038/327031a0
- Tsai, L.H., Harlow, E. and Meyerson, M. (1991) Isolation of the human cdk2 gene that encodes the cyclin Aand adenovirus E1A-associated p33 kinase. Nature, 353, 174- 177. doi:10.1038/353174a0
- Allende, J.E. and Allende, C.C. (1995) Protein kinases. 4. Protein kinase CK2: An enzyme with multiple substrates and a puzzling regulation. FASEB Journal, 9, 313-323.
- Derijard, B., Hibi, M., Wu, I.H., et al. (1994) JNK1: A protein kinase stimulated by UV light and Ha-Ras that binds and phosphorylates the c-Jun activation domain. Cell, 76, 1025-1037. doi:10.1016/0092-8674(94)90380-8
- Cantley, L.C. (2002) The phosphoinositide 3-kinase pathway. Science, 296, 1655-1657. doi:10.1126/science.296.5573.1655
- List of SCADS inhibitor kit III (2012). http://gantoku-shien.jfcr.or.jp/kit3.xls
- Ushijima, H. and Maeda, M. (2012) cAMP-dependent proteolysis of GATA-6 is linked to JNK-signaling pathway. Biochemical and Biophysical Research Communications, 423, 679-683. doi:10.1016/j.bbrc.2012.06.013
- Huggon, I.C., Davies, A., Gove, C., et al. (1997) Molecular cloning of human GATA-6 DNA binding protein: High levels of expression in heart and gut. Biochimica et Biophysica Acta, 1353, 98-102. doi:10.1016/S0167-4781(97)00049-3
- Suzuki, E., Evans, T., Lowry, J., et al. (1996) The human GATA-6 gene: Structure, chromosomal location, and regulation of expression by tissue-specific and mitogenresponsive signals. Genomics, 38, 283-290. doi:10.1006/geno.1996.0630
- LaVoie, H.A., McCoy, G.L., Blake, C.A. (2004) Expression of the GATA-4 and GATA-6 transcription factors in the fetal rat gonad and in the ovary during postnatal development and pregnancy. Molecular and Cellular Endocrinology, 227, 31-40. doi:10.1016/j.mce.2004.07.016
- Visvikis, O., Lorès, P., Boyer, L., et al. (2007) Activated Rac1, but not the tumorigenic variant Rac1b, is ubiquitinated on Lys 147 through a JNK-regulated process. FEBS Journal, 275, 386-396. doi:10.1111/j.1742-4658.2007.06209.x
- Chuang, J.Y., Wang, Y.T., Yeh, S.H., et al. (2008) Phosphorylation by c-Jun NH2-terminal kinase 1 regulates the stability of transcription factor Sp1 during mitosis. Molecular Biology of the Cell, 19, 1139-1151. doi:10.1091/mbc.E07-09-0881
- Baker, S.J., Kerppola, T.K., Luk, D., et al. (1992) Jun is phosphorylated by several protein kinases at the same sites that are modified in serum-stimulated fibroblasts. Molecular and Cellular Biology, 12, 4694-4705.
- Bousset, K., Oelgeschläger, M.H., Henriksson, M., et al. (1994) Regulation of transcription factors c-Myc, Max, and c-Myb by casein kinase II. Cellular & Molecular Biology Research, 40, 501-511.
- Sreedhar, A.S., Soti, C. and Csermely, P. (2004) Inhibition of Hsp90: A new strategy for inhibiting protein kinases. Biochimica et Biophysica Acta, 697, 233-242.
NOTES
*Corresponding author.

