Journal of Cancer Therapy
Vol. 4 No. 7 (2013) , Article ID: 36240 , 14 pages DOI:10.4236/jct.2013.47140
Ductal Carcinoma in Situ Treatment Requires a Multidisciplinary Approach
![]()
Research Oncology, Bermondsey Wing, Guy’s Hospital, London, UK.
Email: *ian.fentiman@gstt.nhs.uk
Copyright © 2013 Chloe Constantinou, Ian S. Fentiman. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received June 25th, 2013; revised July 24th, 2013; accepted July 30th, 2013
Keywords: ductal carcinoma in situ; epidemiology; molecular profile; surgery; radiotherapy; tamoxifen
ABSTRACT
Correct diagnosis and treatment are crucial for DCIS because it is a direct precursor of potentially lethal invasive breast cancer (IBC). As a result of mammographic screening, the incidence of DCIS rose from 1.87% per 100,000 women from 1973-1975 to 32.5% per 100,000 in 2005. The incidence of DCIS is strongly associated with advanced age, an older age at the time of the first birth or nulliparity, family history of a first-degree relative with BC, BRCA1 and BRCA2 mutation carriers, history of biopsy, late age at menopause, and elevated body mass index, the use of HRT over 5 years. With the use of screening mammography, eight population-based trials showed an increase in DCIS incidence reaching 20% with significant reductions in breast cancer mortality. MRI is also used in combination with the mammography for the diagnosis of DCIS. Three grades of DCIS are ultimately recognized: grade 1/low grade, grade 2/intermediate grade, and grade 3/high grade. Several options are available for the management of DCIS, including breast-conserving surgery, with or without postoperative radiotherapy, and with the clear margin being the most important factor for reducing risk of local recurrence. A 2 mm margin is superior to <2 mm, but there was no significant difference in relapse rate in those with margins of 2 or 5 mm when combined with radiotherapy. The use of mastectomy for treatment of DCIS has declined steadily. Sentinel lymph node biopsy (SLNB) should be performed on patients undergoing mastectomy for DCIS, and a case-by-case decision should be made to perform SLNB in patients who have a high risk DCIS or large tumours. Prospective and retrospective studies have demonstrated excellent long-term results after BCS and radiotherapy, as opposed to BCS alone that has shown a higher rate of local recurrence. Tamoxifen also reduces ipsilateral and contralateral breast cancer events in women with DCIS and is the only systemic therapy approved by Food Drug Administration for this disease. Aromatase inhibitors and other targeted therapies are currently being evaluated in ongoing studies.
1. Introduction
The human breast comprises thousands of lobules, interconnected by small ducts, which join to form larger ducts that carry milk to the nipple. Ductal Carcinoma in Situ (DCIS) describes lesions characterised by the proliferation of abnormal epithelial cells but without evidence of invasion through the basement membrane into the surrounding stroma. Since DCIS represents local disease without regional involvement, it is not considered lifethreatening. Nonetheless, correct diagnosis and treatment are essential since DCIS is a direct precursor of potentially lethal invasive breast cancer (IBC). During the latter half of the 20th century, as a result of early diagnosis by screening mammography and results of several randomized controlled trials (RCTs) of therapies for DCIS there was a change in perception of the nature and treatment of DCIS [1-10].
2. Epidemiology
As a result of screening mammography, the incidence of DCIS rose from 1.87/100,000 women in 1973-1975 to 32.5/100,000 in 2005. This increase occurred in all age groups. There was a fivefold increase between 1983 and 2003 among women aged ≥50 years and older, which started to decline in 2003, possibly because of the reduced use of hormone replacement therapy. In women <50 years there was an almost threefold increase which continues to rise [11-13] . The “epidemic” of DCIS has not been uniform across histological types: comedo DCIS remained constant or decreased whereas non-comedo DCIS is diagnosed more frequently across all age groups [12].
There is a strong association with advanced age, peaking at 65 - 69 years and declining slowly by the age of 79. DCIS is extremely uncommon before the age of 35 - 39 [14,15] . There is a similar incidence among white, African American and Asian/Pacific Islanders [16,17] . As for IBC, risk factors for DCIS include older age at first birth, nulliparity, family history of a first-degree relative with BC, BRCA1 and BRCA2 mutation carriers, history of biopsy, late age at menopause, and elevated body mass index [18-24] . Furthermore, increased mammographic breast density, is also associated with an increased risk of DCIS; women with density >45 cm2 have greater odds for developing DCIS than those with density less than 15 cm2. No association has been found between current or past use of the oral contraceptive pill and DCIS [20,22,23, 26-28] . In contrast, a large prospective cohort study in the UK found a 56% increased risk of DCIS among current users of HRT compared to never users [29].[29] Other studies reported that DCIS risk was associated with the duration of HRT use; those presently using it for <5 years have a significantly reduced risk of DCIS than the never users, whereas those currently on HRT for >5 years had a greater risk than the never users [30,31] . The Women’s Health Initiative randomized trial found no increased risk of DCIS associated with HRT [32] .
In a cohort study with 486 cases of DCIS, Kabat et al. reported no increased risk of DCIS in postmenopausal smokers [33]. Lactation, early menarche and increased alcohol consumption were not associated with an increased risk of DCIS. Exercise for >4 hours may diminish the risk for DCIS [34]. This was confirmed in a second study of 1925 DCIS survivors in whom the risk of a second breast diagnosis was reduced by increasing physical activity and reducing alcohol consumption [35].
3. Diagnosis
3.1. Mammography and DCIS
In the pre-mammography era, DCIS was only 1% - 2% of breast cancers, presenting as a large palpable lesion with mastectomy was the standard therapy [36]. Subsequently 8 population-based trials showed an increase in DCIS incidence reaching 20% with significant reductions in breast cancer mortality [37-44]. The sensitivity of mammography in the detection of DCIS is 86%, with 80% - 85% of DCIS detected by mammography and the remaining 15% clinically detected as a lump [14,45]. DCIS usually appears as calcifications which can take on a range of shapes, including amorphous, coarse, fine pleomorphic, and fine linear calcifications. They can also and these can be linked with disease biology; fine-linear/ linear-branching calcifications are being investigated as a factor of poorer prognosis [46]. Mammographic detection of non-palpable and smaller DCIS lesions allowed breast-conserving surgery (BCS) also to be considered as a treatment option.
3.2. MRI and DCIS
The use of MRI for DCIS has prompted recommendations as to its use. There are two main indications: determination of extent of disease as mammography may underestimate the size due to non-calcified lesions, and detection of DCIS in high-risk women who may have mammographically occult lesions [47]. Contrast-enhanced 3D T1-weighted images are used to evaluate DCIS which usually appears as non-mass-like enhancement, with clumped internal enhancement in a segmental, linear or regional distribution [48-52].
The sensitivity of MRI compared to mammography has been investigated in several studies and been found to be higher in the detection of DCIS [53,54]. Screening trials also confirmed the higher sensitivity of MRI, especially for high-grade lesions [55,56] but others did not concur, possibly dependent upon the experience of the radiologist [57-59].
Unfortunately, there are some limitations as to the use of MRI. Whereas a mammogram can underestimate extent an MRI can overestimate significantly. Recent studies report 40% false negative results for DCIS [52,53, 60-63]. The proportion of BRCA-positive DCIS detected on MRI is only 10%, probably because BRCA-positive cancers may be more aggressive and also, as suggested by Warner et al., due to the learning curve of the radiologist [64,65]. Furthermore, the benefit of obtaining free surgical margins with the use of MRI has yet to be established [66-69]. Solin et al. in a study of 136 DCIS found no improvement in re-excision or local recurrence rates among patients undergoing pre-operative MRI [69]. Similarly, Pilewskie et al. in a study of 352 DCIS patients, 217 of whom underwent MRI, reported that the type of initial operation and number of re-operations were similar for the 2 groups and MRI was not superior to mammography for determination of DCIS extent preoperatively [70].
4. Pathology
Historically, DCIS has been classified according to the predominant architectural microscopic pattern of the proliferation. This classification includes comedo, cribriform, micropapillary, solid, and papillary subtypes. In the premammography era, the comedo DCIS was the most common type, usually comprising large, irregularly shaped, rapidly dividing cells growing as a palpable mass with a necrotic centre. Other types were rarely found before the diagnostic mammogram was established, as they are seldom palpable or symptomatic and the cells are smaller, of more normal appearance and less necrotic than comedo DCIS. However, a system of categorization based on the growth pattern alone is problematic as 62% of DCIS lesions tend to have a mixture of architectural patterns [71]. Hence, newer systems of taxonomy are based on nuclear grade and evolved to reflect differentiation and growth. Three grades are ultimately recognized: grade 1/low grade, grade 2/intermediate grade, and grade 3/high grade [72,73] . (Table 1) Grading of DCIS can be helpful in estimating the risk of local recurrence and is also relatively reproducible. A core biopsy showing highgrade DCIS represents a 48% risk of the presence of a radiologically occult invasive focus; in order to avoid a second surgical procedure, the patient could benefit from sentinel lymph node biopsy combined with a wide local excision of DCIS [74].
Following a full pathological review of 72% of the 1694 cases entered into the UKCCCR/ANZ DCIS trial, Pinder et al. [75] proposed a new pathological classification for DCIS with substantially better prognostic discrimination for ipsilateral recurrence than the classical categorization based on cytonuclear grading alone. They identified a group of patients with a particularly poor outcome: women who had DCIS not only of high nuclear grade, but also of pure (>50%) solid architecture with extensive necrosis (>50% of ducts) displayed a significantly worse outcome than those with a high cytonuclear grade alone. The new mode of classification (Table 2) can help to identify women of low risk that would gain no benefit from any further adjuvant therapy, and those
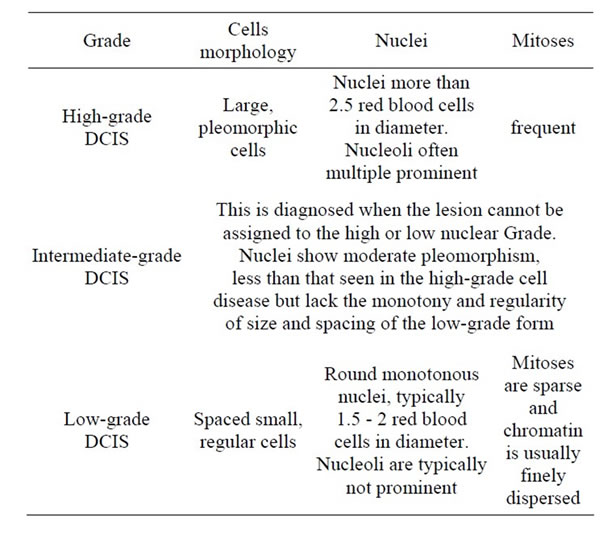
Table 1. DCIS histological grades [145].

Table 2. Pinder et al. proposed system of classification for DCIS [76].
at high risk who would require maximal local therapy. More large series are needed to validate these findings [76].
5. Molecular Biology
Many studies have compared the gene expression, genetic and epigenetic profiles of DCIS and invasive breast carcinomas in order to identify diagnostic markers that differentiate between in situ and invasive tumours, and predictive markers that correlate with the risk of invasive progression [77]. In situ and invasive carcinomas of the same histological subtype share the same genetic and epigenetic alterations, unlike luminal A, luminal B, HER2+ and basal-like molecular profiles that are completely different. Mutations in numerous oncogenes and suppressors, including TP53, PTEN, PIK3CA, ErbB2, and MYC, have been analyzed in IDC and DCIS by several comparative studies. Differences in the frequency of these changes have been found according to the tumour subtype but not histological stage; HER2 and basal-like subtypes displayed TP53 mutation more frequently than the luminal type, whereas loss of PTEN and amplification of ErbB2 was more specific for HER2 subtypes [78]. In a study of 236 DCIS patients treated with breast-conserving surgery, Knudsen et al. [79] examined the association of two major tumour suppressor genes—retinoblastoma (RB) and phosphatase and tensin homolog (PTEN)—and the risk for ipsilateral breast event (IBE) or progression to invasive breast cancer (IBC). Loss of RB immunoreactivity in DCIS was strongly associated with the risk of IBE occurrence and IBC recurrence. PTEN loss occurred frequently in DCIS but was not associated with recurrence or progression. However, patients with DCIS lesions that were both RB and PTEN-deficient were at further risk of IBEs which indicates that RB and PTEN together can be used as a prognostic marker and also as aggressive target treatment ADDIN EN.CITE ADDIN EN.CITE.DATA [79] . Pandey et al. [80] examined the expression of key lipogenic genes, including ACLY, ACC1 and FAS, in 111 clinical samples of DCIS and found that these genes were significantly up-regulated in all grades of DCIS compared to normal breast tissue. They have also shown that in animals, the inhibition of lipogenic gene expression in cancer stem-like cells (CSCs) with resveratrol suppressed their ability to generate DCIS [80].
Comparative genomic hybridization (CGH)-based analysis of DCIS and invasive carcinoma performed by Buerger et al. revealed that losses of 16q were seen almost exclusively in lowand intermediate-grade DCIS, whereas a higher frequency of 1q gain and 11q loss was observed in intermediate-grade DCIS [81] . However, highgrade DCIS demonstrated complex genomic alterations characterized by loss of 8p, 11q, 13q, and 14q, gains of 1q, 5p, 8q, and 17q, and by high-level amplifications of 17q 12 and 11q 13 [81] . Moreover, an analysis of CGH data generated from synchronous and metachronous IDC and DCIS lesions revealed a near-identical pattern of genetic change, supporting a direct precursor relationship between DCIS and IDC [81-83] .
Gautherier et al. reported that high expression of COX-2 and Ki67 in DCIS correlated with higher risk of local recurrence of in situ or invasive carcinoma [84] . Additionally, Lu et al. demonstrated a functional cooperation between ErbB2 and 14-3-3ζ that may increase the risk of invasive progression by promoting the epithelial to mesenchymal transition [85] .
Micro RNAs might be used as novel biomarkers for the diagnosis of early breast cancer as they increase with tumour progression [86,87] . The analysis of the expression of miR-21, a microRNA and its targets (PTEN, PDCD4 and TMI) in a normal breast and in DCIS and IDC showed a gradual increase in miR-21 expression during tumorigenesis [86] . Another independent study by Sempere et al. also found higher miR-21 expression with tumour progression along with increased miR-45 in DCIS compared with atypical hyperplasia [87].
The increase of tumour invasiveness by the loss of CD34 expression from the CD34 fibrocytes of normal mammary stroma and the acquisition of α-smooth muscle actin (SMA) expression was examined by Catteau et al. [88] who found them to be more frequent in high-grade in situ lesions than in intermediate and low-grade lesions (p < 0.001); the loss of CD34 was even higher in the presence of necrosis. The SMA expression was not associated with necrosis [88].
The association of DCIS with triple negative invasive breast cancer (TNBC) was examined by Aye et al. after evaluating 241 invasive TNBC, of which 62.6% in situ lesions were of high nuclear grade and 68% revealed basal-like expression of both in-situ and invasive components of the same case. These data support the thesis that triple negative carcinoma in situ is the precursor of the corresponding invasive counterpart and that the basallike phenotype in the majority of cases harbours basallike in situ to basal-like invasive BC.
The limitation of all the above-mentioned studies is the small population of patients. In contrast to the significant progress made in the molecular-based classification of invasive breast cancer, the management of DCIS is based on histopathological findings due to the small number of DCIS samples from large cohorts of uniformly treated patients with long-term clinical follow-up. Recently, Solin et al. reported a series of 327 patients with DCIS treated by surgical excision without radiation within the Eastern Cooperative Oncology Group (ECOG) E5194 study. [89] associated with the risk of developing an ipsilateral breast event. They identified low, intermediate, and high groups, with 10-year risks of developing IBC being 4%, 12%, and 19%, respectively. Using this approach it may be possible to use gene expression to determine need for additional surgical or systemic therapy.
6. Treatment
Several options for the management of DCIS include breast-conserving surgery (BCS), with or without postoperative radiotherapy, and mastectomy. Achieving a clear margin is the most important determinant of the treatment as it constitutes a major risk factor for local recurrence.
6.1. Breast Conserving Surgery
Several randomized clinical trials in Europe and North America have evaluated BCS with or without radiotherapy for patients with DCIS (Table 3). Lumpectomy alone, without radiotherapy, resulted in consistently higher rates of local recurrence, (8% - 34% vs. 0% - 17%) respectively. The most common risk factors for local recurrence involved margins, young age and high-grade tumours with comedo necrosis. There was a notable variation among studies in terms of the optimal width of a negative margin. In a meta-analysis [90] of BCS for DCIS studies (1970-2010), negative margins as opposed to positive margins, with or without radiotherapy, were associated with a reduced risk of IBR (ipsilateral breast recurrence). Compared with a negative margin >2 mm, a negative margin of at least 10 mm was associated with a lower risk of IBR. Wang et al. concluded that the surgeon must try to achieve margins as wide as possible but of course such an approach will lead to poor cosmetic outcomes for many patients. Another meta-analysis by Dunne et al. examined margin width and risk of IBR in patients with pure DCIS treated by wide excision and radiotherapy. A negative margin was associated with a significant reduction in IBR (OR 0.36, 95 & CI 0.27-

Table 3. Randomised trials of radiotherapy in DCIS.
0.47). A 2 mm margin was superior to <2 mm, but there was no significant difference in relapse rate in those with margins of 2 or 5 mm when combined with radiotherapy [91]. Silverstein et al. examined 496 specimens of DCIS and concluded that there was no benefit to be gained from adjuvant radiotherapy in patients that had margin widths of 1 - 10 mm or >10 mm, whereas the rate of local recurrence was lower among patients with margin <1 mm after adjuvant radiotherapy [9] .
Owing to the expense, inconvenience and potential adverse effects of radiotherapy, its routine use for low grade DCIS after lumpectomy has been questioned. Hence, in 2008, the National Comprehensive Cancer Network included excision alone as an acceptable treatment choice for DCIS, but without defining which group of patents is eligible for such treatment. Some groups have developed grading systems with the most popular being Van Nuys Prognostic index (VNPI), developed by Silverstein et al. in 1995 [92] . VNPI is an algorithm that quantifies five measurable prognostic/risk factors for local recurrence [93] (Table 4). Initially, this index only used nuclear grade and necrosis as predictor factors; in 1996, size and margin were added, and age was later to be included in 2002. If excision alone is to achieve a local recurrence rate of less than 20%, the patients concerned must have a score of 4, 5, or 6; margins >3 mm are required in patients with a score of 7.
Once the choice of BCS is made, the patient must be made aware of the possibility that for an extended period she will undergo diagnostic mammograms and invasive procedures in the conserved breast. In a study by Nekhlyudov et al. of 2948 women with DCIS treated with BCS, 907 (30.8%) were submitted to 1422 diagnostic mammograms, and 1813 (61.5%) underwent 2305 ipsilateral invasive procedures [94] . The estimated 10-year cumulative risk of having at least one diagnostic mammogram after initial DCIS excision and one invasive procedure was 41% and 66% respectively ADDIN EN.CITE ADDIN EN.CITE.DATA [94] .
6.2. Mastectomy
The use of mastectomy for treatment of DCIS has declined steadily. Ernster et al. evaluated the treatment of DCIS using Surveillance Epidemiology and End Results data from 1973 to1992 and found that the number of patients treated with mastectomy fell from 71% in 1983 to 44% in 1992 [11] . Mastectomy usually advised for the following indications: extensive and/or multifocal DCIS involving 4 - 5 cm for disease or more than one quadrant,

Table 4. Van nuys prognostic index [93] .
inability to obtain tumour-free margins by lumpectomy and/or re-excision(s), patients in whom breast irradiation is potentially contraindicated or who lack access to irradiation, suboptimal tumour to breast size ratio, where a margin-negative lumpectomy will yield an unacceptable cosmetic result (as defined by the patient), and sometimes the patient’s strong preference for mastectomy. There are various surgical approaches to mastectomy for DCIS: simple mastectomy (excision of the breast tissue and overlying skin), skin-sparing mastectomy (removal of the breast with preservation of the skin envelope) and nipple-preserving mastectomy techniques. The risk for local recurrence in these three approaches is very low, provided a complete excision of the breast tissue is performed [36,95,96] . The low recurrence reported for the nipple-sparing mastectomy may be due to the fact that DCIS rarely involves the nipple-areola complex as opposed to invasive ductal carcinoma and invasive lobular carcinoma [97], and the exclusion of patients with centrally located disease, extensive DCIS or radiographic abnormalities in proximity to the nipple-areolar complex.
Clinical trials and population-based studies with longterm follow up report excellent clinical outcomes following mastectomy for DCIS with a 1% - 2% rate of local recurrence (LR) [98,99] . The majority of LRs are detected on palpation [36]. In a series of 80 DCIS patients who had undergone mastectomy, Rashtian et al. [100] reported six (7.5%) with LR at median follow-up of 61 months. This was associated with high grade and margins of less than 1mm. In another small review, 10 chest wall recurrences were associated with young age and multifocality [101]. Women with isolated locoregional recurrence after mastectomy displayed long-term disease-free survival after surgical excision combined with chest wall radiotherapy [101].
To date, no prospective randomized trial has directly compared mastectomy and lumpectomy. In the NSABP B-06 trial [102] (Table 3), 78 DCIS were incidentally included; the overall survival between mastectomy/BCS/ BCS + radiation was similar. Local recurrence was higher for BSC alone (43%) but decreased after the addition of radiotherapy (7%). Although the LR risk is low, there is a difference in the long-term outcome in terms of progression to invasive cancer, with a two-fold higher mortality rate.
Immediate breast reconstruction is often offered after mastectomy to DCIS patients as these patients rarely undergo radiotherapy post mastectomy. In a study of 238 patients treated with radical mastectomy for DCIS, 57.1% had immediate breast reconstruction and 42.9% no reconstruction [103]. The main reason for a patient not having reconstruction was simply because it was not offered by the surgeon [103].
6.3. Sentinel Lymph Node Biopsy (SLN) and Management of the Axilla in Ductal Carcinoma in Situ
DCIS does not cause invasion and metastasis. Axillary lymph node dissection (ALND) is no longer indicated for DCIS patients. Silverstein et al. [104] reported a study of 100 patients treated with mastectomy (n49) or BCS (n51) and ALND, all of whom had negative lymph nodes. In a review of the NSABP B-17 and NSABP B-24 trial, the risk of axillary lymph node recurrence was less than 1% [105] . Similarly, a low axillary recurrence was reported by the City of Cancer Center after a long-term follow-up of DCIS patients treated with lumpectomy and whole breast irradiation [106].
Theoretically, sentinel lymph node biopsy (SLN) does not appear to be indicated in patients with pure DCIS. However, about 15% of patients who are preoperatively diagnosed with DCIS on core-needle biopsy were upstaged postoperatively to microinvasive (extension of the cancer cells beyond the basement membrane into adjacent tissues with focus no more than 1 mm in greatest dimension) or invasive disease in the final pathology report, thereby eliminating the benefit of a one-stage procedure [107-110] . The question that arises is which patients with an initial diagnosis of DCIS present a high possibility of having invasive or microinvasive disease and should consequently undergo axillary evaluation with SLN?
Multiple investigators have stressed the need for SLN in patients with high-risk DCIS in order to rule out invasive disease and also as not to operate on patients twice [111-113] . Among the independent predictor factors that increase the probability of DCIS being upstaged to microinvasive or invasive disease are: patients aged 55 years or under, mammographic abnormality greater than 4 cm, high grade of DCIS or comedo necrosis on histological evaluation, and the presence of a palpable tumour [114-116] .
Conversely, other investigators fail to see any benefit in subjecting the patients to SLN which can cause troublesome morbidities (lymphoedema, pain, nerve injury, paraesthesia, numbness, decreased limb use, and shoulder dysfunction) unless invasive disease is confirmed with core biopsy or final histology [117-119] . As shown in studies of patients with DCIS or microinvasion by both Murphy et al. [119] and Lara et al. [120] , a positive SLN was not associated with a high risk of local or distant recurrence.
Performing SLN at mastectomy has also been evaluated [111,112,114,121] . Those same indications that favour mastectomy in these patients can also be considered as risk factors for invasive or microinvasive disease and that SLN is less efficacious after mastectomy [122] lend credence to SLNB being a reasonable procedure to carry out before or during mastectomy. In conclusion, SLNB should be performed on patients undergoing mastectomy for DCIS, and a case-by-case decision should be made to perform SLNB in patients who have a high risk DCIS or large tumours.
6.4. Role of Radiotherapy in the Management of DCIS
As most women with DCIS are interested in breast conserving treatment, a major decision for these women is whether or not to add radiotherapy. As mentioned above, four prospective randomized clinical trials measured the value of adding radiation after BCS for DCIS (Table 3) [123-130] . A meta-analysis of these trials yielded a total of 3729 women were randomized either to receive radiation after BCS or not. The 10-year rate for ipsilateral LR (IBLR) (invasive carcinoma plus DCIS) decreased by 15% with the addition of radiotherapy. Additionally, the 10-year rate of invasive and DCIS local failure was reduced 9% and 8% respectively. There were no differences in the 10-year overall survival rate (8.2% vs. 8.4% respectively), mortality without recurrence (5.7% vs. 5.4% respectively) or cardiac mortality (most important when evaluating a patient with left-sided DCIS for radiation treatment) [131]. Another meta-analysis of these randomized trials by EBCTCG demonstrated that only a very large randomized clinical trial or meta-analysis would have sufficient statistical power to determine whether any survival benefit was to be gained from the addition of radiotherapy [132] .
In a study by Solin et al. [133] , 1003 women with unilateral, mammographically detected DCIS were treated with lumpectomy followed by radiotherapy. The median follow-up was 8.5 years, and the mean patient age was 5 years. The 15-year overall survival rate was 89%, the 15- year cause-specific survival rate was 98%, and the 15- year distant metastases-free survival was 97% with most patients having died of causes unrelated to the breast cancer. The 15-year rate of LR (DCIS or IBC) was 19%. Patient age >50 years at the time of treatment and negative margins were both independently associated with a decreased risk of local failure, with LR in these two groups being ≤8% ADDIN EN.CITE ADDIN EN.CITE.DATA [133] .
Rakovitch et al. [134] analysed 3762 DCIS patients treated with BCS, 1895 of whom also received radiotherapy. At 10-year median follow-up, the actuarial rate of LR in women receiving radiotherapy was 13% as compared with 20% in those not submitted to irradiation. It was estimated that 22% of LR could have been avoided had radiotherapy been added.
The ECOG E5194 study had 2 arms: 1) lowor intermediate-grade DCIS 2.5 cm in size or less (565 pt), and 2) high-grade DCIS 1cm or less (105 pt). A minimum 3 mm negative margin was required and no radiotherapy was given. At median 6.7-year follow-up, the 5- year rate and 7-year rate of LR for HG DCIS stood at 15% and 18% respectively compared with 6% and 11% at 6.2-year follow-up for low and intermediate grade DCIS. These data suggest that patients with HG DCIS are not suitable for BCS alone [135] . These results are consistent with another study by Balleine et al. with 134 patients [136].
Prospective and retrospective studies have demonstrated excellent long-term results after BCS and radiotherapy, as opposed to BCS alone that has shown a higher rate of local recurrence. Following a multivariate analysis, age was found to be a significant variable associated with LR in HG DCIS [135] . Margin width is also an important variable, with the minimum negative margin being 10 mm [9] for BCS alone, as compared to a 2 mm minimum margin that is recommended if BCS is combined with radiotherapy [91]. Additional markers are needed to identify a low-risk group in whom radiation can be safely given.
6.5. The Role of Systemic Therapy in Ductal Carcinoma in Situ
6.5.1. Tamoxifen
Two randomized phase III trials have been conducted to assess the efficacy of Tamoxifen after BCS, with or without radiotherapy, to reduce recurrence (Table 3). In the National Surgical Adjuvant Breast and Bowel Project (NSABP) B-24 trial [125,137] , 1804 women with DCIS were randomly given Tamoxifen or placebo for five years. Those eligible had also received radiotherapy. At 12-year follow-up, Tamoxifen had reduced the risk of all breast cancer LR by 31% (ipsilateral invasive breast cancer by 31%, contralateral breast cancer events by 43%). Similar benefits applied to women under 50 years of age or older. Overall survival did not differ between the Tamoxifen and the placebo group. ER status was not analyzed initially, but in a retrospective analysis evaluating ER status in 732 cases, 76% proved to be ER-positive. The risk of recurrence for invasive breast cancer was reduced by 40% in ER-positive women compared to the placebo group.
In the United Kingdom/Australia/New Zealand (UK/ ANZ) trial [130] (Table 3), at 52-month median followup 1701 patients showed a statistically significant reduction of 32% in ipsilateral and contralateral non-invasive cancers in the Tamoxifen vs. non-Tamoxifen groups. In the group receiving radiotherapy, the addition of Tamoxifen made no statistically significant difference to invasive or non-invasive breast cancers. As concerns the side effects, Tamoxifen was well-tolerated; serious and adverse events (thrombo-embolic disease and endometrial cancer) are rare and age-related [138] .
Given the low rate of recurrence, few DCIS patients become candidates for Tamoxifen after mastectomy. Further studies with an adequate selection of patients will help to identify those who could really benefit from receiving Tamoxifen.
6.5.2. Aromatase Inhibitors
NSABP B-35 trial and International Breast cancer Intervention Study-II (IBIS-II) are currently evaluating the role of Anastrozole as adjuvant therapy for patients with DCIS. In the NSABP B-35 trial, post menopausal women with ER-positive and/or progesterone receptor-positive DCIS treated with lumpectomy and radiotherapy are randomized to receive Anastrozole or placebo and Tamoxifen or placebo for a period of five years. The IBIS-II trial is also evaluating Anastrozole and Tamoxifen, but not always combined with radiotherapy. Aromatase inhibitors are also being investigated in a neoadjuvant setting in a pilot study of Tamoxifen or Letrozole in 40 women with ER (+) DCIS. The response to the endocrine therapy that commences 3 months prior to surgery is evaluated with mammograms, MRI and tissue biomarkers [139,140]. Bundred and colleagues randomized 90 postmenopausal women with ER-positive DCIS to receive Exemestane or placebo in a preoperative, 14-day, window-of-opportunity trial. The response and impact on KI-67 was evaluated, and Exemestane was shown to have reduced Ki67 by 9% (p < 0.001) [141].
6.5.3. Other Targeted Therapy
A number of other molecular targets are under investigation with regard to DCIS (Table 5). Pure DCIS is more likely to express HER2/neu than invasive breast cancer [142]. Farnie et al. cultured two DCIS cell lines, MCF10 DCIS.com (ErbB2-normal) and SUM225 (ErbB2-overexpressing), and seven human primary DCIS samples in the presence, absence or combination of the Notch inhibitor, DAPT and ErbB1/2 inhibitors, Lapatinib or Gefitinib. Combined DAPT/Lapatinib treatment emerged as more effective at reducing acini size in both DCIS cell lines than monotherapy, regardless of ErbB2 status. Targeted therapies combining Notch and ErbB1/2 inhibitors should be investigated regardless of ErbB2 receptor status [143]. Cycloxygenase (COX-2) expression is also common in invasive carcinomas and DCIS [144].
7. Conclusion
DCIS is not life-threatening since it represents local disease with no regional involvement. However, being an immediate precursor of potentially lethal invasive breast cancers, its diagnosis and treatment command a multidisciplinary approach. With the use of mammography and MRI, the extent of DCIS can be assessed and it can guide the surgeon to decide whether BCS or mastectomy is indicated for complete removal of the disease. The type of surgery and the pathology report detailing the grade, size, width of clear margin, and also the age of the patient will help the oncologist decide on the appropriate adjuvant treatment for the patient. Radiotherapy has been found to significantly reduce the risk of LR after BCS, especially

Table 5. Targeted therapies for DCIS.
if the clear margin is 2 mm or less. Tamoxifen also reduces ipsilateral and contralateral breast cancer events in women with DCIS and is the only systemic therapy approved by Food Drug Administration for this disease. Aromatase inhibitors and other targeted therapies are currently being evaluated in ongoing studies.
REFERENCES
- G. W. Chang, D. C. Allred, S. K. Mohsin and S. A. Fuqua, “Histological and Biological Evolution of Human Premalignant Breast Disease,” Endocrine-Related Cancer, Vol. 8, No. 1, 2001, pp. 47-61. doi:10.1677/erc.0.0080047
- S. J. Schnitt, et al., “Ductal Carcinoma in Situ (Intraductal Carcinoma) of the Breast,” New England Journal of Medicine, Vol. 318, No. 14, 1988, pp. 898-903. doi:10.1056/NEJM198804073181406
- G. S. Dalgin, et al., “Portraits of Breast Cancer Progression,” BMC Bioinformatics, Vol. 8, 2007, p. 291. doi:10.1186/1471-2105-8-291
- B. Erbas, et al., “The Natural History of Ductal Carcinoma in Situ of the Breast: A Review,” Breast Cancer Research and Treatment, Vol. 97, No. 2, 2006, pp. 135- 144. doi:10.1007/s10549-005-9101-z
- H. M. Kuerer, et al., “Ductal Carcinoma in Situ: State of the Science and Roadmap to Advance the Field,” Journal of Clinical Oncology, Vol. 27, No. 2, 2009, pp. 279-288. doi:10.1200/JCO.2008.18.3103
- K. Polyak, “Breast Cancer: Origins and Evolution,” Journal of Clinical Investigation, Vol. 117, No. 11, 2007, pp. 3155-3163. doi:10.1172/JCI33295
- M. Morrow, “Magnetic Resonance Imaging in the Preoperative Evaluation of Breast Cancer: Primum Non Nocere,” Journal of the American College of Surgeons, Vol. 198, No. 2, 2004, pp. 240-241. doi:10.1016/j.jamcollsurg.2003.10.013
- V. L. Ernster and J. Barclay, “Increases in Ductal Carcinoma in Situ (DCIS) of the Breast in Relation to Mammography: A Dilemma,” Journal of the National Cancer Institute Monographs, Vol. 22, 1997, pp. 151-156.
- M. J. Silverstein, et al., “The Influence of Margin Width on Local Control of Ductal carcinoma in Situ of the Breast,” New England Journal of Medicine, Vol. 340, No. 19, 1999, pp. 1455-1461. doi:10.1056/NEJM199905133401902
- L. J. Solin, et al., “Fifteen-Year Results of Breast-Conserving Surgery and Definitive Breast Irradiation for the Treatment of Ductal Carcinoma in Situ of the Breast,” Journal of Clinical Oncology, Vol. 14, No. 3, 1996, pp. 754-763.
- V. L. Ernster, et al., “Incidence of and Treatment for Ductal Carcinoma in Situ of the Breast,” Journal of the American Medical Association, Vol. 275, No. 12, 1996, pp. 913-918. doi:10.1001/jama.1996.03530360023033
- C. I. Li, J. R. Daling and K. E. Malone, “Age-Specific Incidence Rates of in Situ Breast Carcinomas by Histologic Type, 1980 to 2001,” Cancer Epidemiology, Biomarkers & Prevention, Vol. 14, No. 4, 2005, pp. 1008- 1011. doi:10.1158/1055-9965.EPI-04-0849
- M. Horner, R. L., Krapcho, et al., “SEER Cancer Statistics Review, 1975-2006,” National Cancer Institute, Bethesda, 2009. http://seer.cancer.gov/csr/1975_2006
- V. L. Ernster, et al., “Detection of Ductal Carcinoma in Situ in Women Undergoing Screening Mammography,” Journal of the National Cancer Institute, Vol. 94, No. 20, 2002, pp. 1546-1554. doi:10.1093/jnci/94.20.1546
- W. M. Randolph, et al., “Regular Mammography Use Is Associated with Elimination of Age-Related Disparities in Size and Stage of Breast Cancer at Diagnosis,” Annals of Internal Medicine, Vol. 137, No. 10, 2002, pp. 783- 790. doi:10.7326/0003-4819-137-10-200211190-00006
- A. Ghafoor, et al., “Trends in Breast Cancer by Race and Ethnicity,” CA: A Cancer Journal for Clinicians, Vol. 53, No. 6, 2003, pp. 342-355. doi:10.3322/canjclin.53.6.342
- K. Kerlikowske, et al., “Differences in Screening Mammography Outcomes among White, Chinese, and Filipino Women,” Archives of Internal Medicine, Vol. 165, No. 16, 2005, pp. 1862-1868. doi:10.1001/archinte.165.16.1862
- K. Syrjakoski, et al., “Population-Based Study of BRCA1 and BRCA2 Mutations in 1035 Unselected Finnish Breast Cancer Patients,” Journal of the National Cancer Institute, Vol. 92, No. 18, 2000, pp. 1529-1531. doi:10.1093/jnci/92.18.1529
- K. S. Reinier, P. M. Vacek and B. M. Geller, “Risk Factors for Breast Carcinoma in Situ versus Invasive Breast Cancer in a Prospective Study of Preand Post-Menopausal Women,” Breast Cancer Research and Treatment, Vol. 103, No. 3, 2007, pp. 343-348. doi:10.1007/s10549-006-9375-9
- K. Kerlikowske, et al., “Comparison of Risk Factors for Ductal Carcinoma in Situ and Invasive Breast Cancer,” Journal of the National Cancer Institute, Vol. 89, No. 1, 1997, pp. 76-82. doi:10.1093/jnci/89.1.76
- J. Wohlfahrt, et al., “A Comparison of Reproductive Risk Factors for CIS Lesions and Invasive Breast Cancer,” International Journal of Cancer, Vol. 108, No. 5, 2004, pp. 750-753. doi:10.1002/ijc.11588
- E. B. Claus, M. Stowe and D. Carter, “Breast Carcinoma in Situ: Risk Factors and Screening Patterns,” Journal of the National Cancer Institute, Vol. 93, No. 23, 2001, pp. 1811-1817. doi:10.1093/jnci/93.23.1811
- A. Trentham-Dietz, et al., “Risk Factors for Carcinoma in Situ of the Breast,” Cancer Epidemiology, Biomarkers & Prevention, Vol. 9, No. 7, 2000, pp. 697-703.
- E. B. Claus, et al., “Prevalence of BRCA1 and BRCA2 Mutations in Women Diagnosed with Ductal Carcinoma in Situ,” Journal of the American Medical Association, Vol. 293, No. 8, 2005, pp. 964-969. doi:10.1001/jama.293.8.964
- J. K. Gill, et al., “The Association of Mammographic Density with Ductal Carcinoma in Situ of the Breast: The Multiethnic Cohort,” Breast Cancer Research, Vol. 8, No. 3, 2006, p. R30. doi:10.1186/bcr1507
- T. B. Vamre, H. Stalsberg and D. B. Thomas, “Extra-Tumoral Breast Tissue in Breast Cancer Patients: Variations with Steroid Contraceptive Use,” International Journal of Cancer, Vol. 118, No. 11, 2006, pp. 2827-5831. doi:10.1002/ijc.21697
- K. Bohlke, et al., “Insulin-Like Growth Factor-I in Relation to Premenopausal Ductal Carcinoma in Situ of the Breast,” Epidemiology, Vol. 9, No. 5, 1998, pp. 570-573. doi:10.1097/00001648-199809000-00018
- E. B. Claus, M. Stowe and D. Carter, “Oral Contraceptives and the Risk of Ductal Breast Carcinoma in Situ,” Breast Cancer Research and Treatment, Vol. 81, No. 2, 2003, pp. 129-136. doi:10.1023/A:1025728524310
- G. K. Reeves, et al., “Hormonal Therapy for Menopause and Breast-Cancer Risk by Histological Type: A Cohort Study and Meta-Analysis,” Lancet Oncology, Vol. 7, No. 11, 2006, pp. 910-918. doi:10.1016/S1470-2045(06)70911-1
- S. M. Gapstur, M. Morrow and T. A. Sellers, “Hormone Replacement Therapy and Risk of Breast Cancer with a Favorable Histology: Results of the Iowa Women’s Health Study,” Journal of the American Medical Association, Vol. 281, No. 22, 1999, pp. 2091-2097. doi:10.1001/jama.281.22.2091
- K. Kerlikowske, et al., “Prognostic Characteristics of Breast Cancer among Postmenopausal Hormone Users in a Screened Population,” Journal of Clinical Oncology, Vol. 21, No. 23, 2003, pp. 4314-4321. doi:10.1200/JCO.2003.05.151
- R. T. Chlebowski, et al., “Influence of Estrogen plus Progestin on Breast Cancer and Mammography in Healthy Postmenopausal Women: The Women’s Health Initiative Randomized Trial,” Journal of the American Medical Association, Vol. 289, No. 24, 2003, pp. 3243-3253. doi:10.1001/jama.289.24.3243
- G. C. Kabat, et al., “Cigarette Smoking in Relation to Risk of Ductal Carcinoma in Situ of the Breast in a Cohort of Postmenopausal Women,” American Journal of Epidemiology, Vol. 172, No. 5, pp. 591-599. doi:10.1093/aje/kwq159
- A. V. Patel, et al., “Lifetime Recreational Exercise Activity and Risk of Breast Carcinoma in Situ,” Cancer, Vol. 98, No. 10, 2003, pp. 2161-2169. doi:10.1002/cncr.11768
- P. A. Newcomb, et al., “Alcohol Consumption before and after Breast Cancer Diagnosis: Associations with Survival from Breast Cancer, Cardiovascular Disease, and Other Causes,” Journal of Clinical Oncology, Vol. 31, No. 16, 2013, pp. 1939-1946.
- G. W. Carlson, et al., “Local Recurrence of Ductal Carcinoma in Situ after Skin-Sparing Mastectomy,” Journal of the American College of Surgeons, Vol. 204, No. 5, 2007, pp. 1074-1078. doi:10.1016/j.jamcollsurg.2007.01.063
- A. B. Miller, et al., “Canadian National Breast Screening Study-2: 13-Year Results of a Randomized Trial in Women Aged 50-59 Years,” Journal of the National Cancer Institute, Vol. 92, No. 18, 2000, pp. 1490-1499. doi:10.1093/jnci/92.18.1490
- A. B. Miller, et al., “The Canadian National Breast Screening Study-1: Breast Cancer Mortality after 11 to 16 Years of Follow-Up. A Randomized Screening Trial of Mammography in Women Age 40 to 49 Years,” Annals of Internal Medicine, Vol. 137, No. 5, 2002, pp. 305-312. doi:10.7326/0003-4819-137-5_Part_1-200209030-00005
- S. Shapiro, “Periodic Screening for Breast Cancer: The HIP Randomized Controlled Trial. Health Insurance Plan,” Journal of National Cancer Institute. Monographs, Vol. 1997, No. 22, 1997, pp. 27-30.
- L. Nystrom, et al., “Long-Term Effects of Mammography Screening: Updated Overview of the Swedish Randomised Trials,” The Lancet, Vol. 359, No. 9310, 2002, pp. 909-919. doi:10.1016/S0140-6736(02)08020-0
- N. Bjurstam, et al., “The Gothenburg Breast Screening Trial,” Cancer, Vol. 97, No. 10, 2003, pp. 2387-2396. doi:10.1002/cncr.11361
- L. Tabar, et al., “The Swedish Two-County Trial Twenty Years Later. Updated Mortality Results and New Insights from Long-Term Follow-Up,” Radiologic Clinics of North America, Vol. 38, No. 4, 2000, pp. 625-651. doi:10.1016/S0033-8389(05)70191-3
- M. M. Roberts, et al., “The Edinburgh Randomised Trial of Screening for Breast Cancer: Description of Method,” British Journal of Cancer, Vol. 50, No. 1, 1984, pp. 1-6. doi:10.1038/bjc.1984.132
- J. Frisell, et al., “Follow-Up after 11 Years—Update of Mortality Results in the Stockholm Mammographic Screening Trial,” Breast Cancer Research and Treatment, Vol. 45, No. 3, 1997, pp. 263-270. doi:10.1023/A:1005872617944
- K. Kerlikowske, et al., “Positive Predictive Value of Screening Mammography by Age and Family History of Breast Cancer,” The Journal of the American Medical Association, Vol. 270, No. 20, 1993, pp. 2444-2450. doi:10.1001/jama.1993.03510200050031
- C. K. Kuhl, “Why Do Purely Intraductal Cancers Enhance on Breast MR Images?” Radiology, Vol. 253, No. 2, 2009, pp. 281-283. doi:10.1148/radiol.2532091401
- C. D. Lehman, “Magnetic Resonance Imaging in the Evaluation of Ductal Carcinoma in Situ,” Journal of National Cancer Institute. Monographs, Vol. 2010, No. 41, 2010, pp. 150-151. doi:10.1093/jncimonographs/lgq030
- M. Mossa-Basha, et al., “Ductal Carcinoma in Situ of the Breast: MR Imaging Findings with Histopathologic Correlation,” Radiographics, Vol. 30, No. 6, 2010, pp. 1673- 1687. doi:10.1148/rg.306105510
- T. Yamada, et al., “Radiologic-Pathologic Correlation of Ductal Carcinoma in Situ,” Radiographics, Vol. 30, No. 5, pp. 1183-1198. doi:10.1148/rg.305095073
- S. A. Jansen, et al., “Pure Ductal Carcinoma in Situ: Kinetic and Morphologic MR Characteristics Compared with Mammographic Appearance and Nuclear Grade,” Radiology, Vol. 245, No. 3, 2007, pp. 684-691. doi:10.1148/radiol.2453062061
- J. H. Menell, et al., “Determination of the Presence and Extent of Pure Ductal Carcinoma in Situ by Mammography and Magnetic Resonance Imaging,” The Breast Journal, Vol. 11, No. 6, 2005, pp. 382-390. doi:10.1111/j.1075-122X.2005.00121.x
- E. L. Rosen, et al., “BI-RADS MRI Enhancement Characteristics of Ductal Carcinoma in Situ,” The Breast Journal, Vol. 13, No. 6, 2007, pp. 545-550. doi:10.1111/j.1524-4741.2007.00513.x
- W. A. Berg, et al., “Diagnostic Accuracy of Mammography, Clinical Examination, US, and MR Imaging in Preoperative Assessment of Breast Cancer,” Radiology, Vol. 233, No. 3, 2004, pp. 830-849. doi:10.1148/radiol.2333031484
- C. K. Kuhl, et al., “MRI for Diagnosis of Pure Ductal Carcinoma in Situ: A Prospective Observational Study,” The Lancet, Vol. 370, No. 9586, 2007, pp. 485-492. doi:10.1016/S0140-6736(07)61232-X
- E. Warner, et al., “Surveillance of BRCA1 and BRCA2 Mutation Carriers with Magnetic Resonance Imaging, Ultrasound, Mammography, and Clinical Breast Examination,” The Journal of the American Medical Association, Vol. 292, No. 11, 2004, pp. 1317-1725. doi:10.1001/jama.292.11.1317
- C. K. Kuhl, et al., “Mammography, Breast Ultrasound, and Magnetic Resonance Imaging for Surveillance of Women at High Familial Risk for Breast Cancer,” Journal of Clinical Oncology, Vol. 23, No. 33, 2005, pp. 8469-8476. doi:10.1200/JCO.2004.00.4960
- M. Kriege, et al., “Efficacy of MRI and Mammography for Breast-Cancer Screening in Women with a Familial or Genetic Predisposition,” The New England Journal of Medicine, Vol. 351, No. 5, 2004, pp. 427-437. doi:10.1056/NEJMoa031759
- M. O. Leach, et al., “Screening with Magnetic Resonance Imaging and Mammography of a UK Population at High Familial Risk of Breast Cancer: A Prospective Multicentre Cohort Study (MARIBS),” The Lancet, Vol. 365, No. 9473, 2005, pp. 1769-1778. doi:10.1016/S0140-6736(05)66481-1
- A. J. Rijnsburger, et al., “BRCA1-Associated Breast Cancers Present Differently from BRCA2-Associated and Familial Cases: Long-Term Follow-Up of the Dutch MRISC Screening Study,” Journal of Clinical Oncology, Vol. 28, No. 36, 2010, pp. 5265-5273. doi:10.1200/JCO.2009.27.2294
- [61] I. M. Obdeijn, et al., “Assessment of False-Negative Cases of Breast MR Imaging in Women with a Familial or Genetic Predisposition,” Breast Cancer Research and Treatment, Vol. 119, No. 2, 2010, pp. 399-407. doi:10.1007/s10549-009-0607-7
- [62] M. D. Schnall, et al., “Diagnostic Architectural and Dynamic Features at Breast MR Imaging: Multicenter Study,” Radiology, Vol. 238, No. 1, 2006, pp. 42-53. doi:10.1148/radiol.2381042117
- [63] A. Shimauchi, et al., “Breast Cancers Not Detected at MRI: Review of False-Negative Lesions,” American Journal of Roentgenology, Vol. 194, No. 6, 2010, pp. 1674-1679. doi:10.2214/AJR.09.3568
- [64] A. Teifke, et al., “Undetected Malignancies of the Breast: Dynamic Contrast-Enhanced MR Imaging at 1.0 T,” Radiology, Vol. 224, No. 3, 2002, pp. 881-888. doi:10.1148/radiol.2243010547
- [65] E. Warner, et al., “Improvement in DCIS Detection Rates by MRI over Time in a High-Risk Breast Screening Study,” The Breast Journal, Vol. 17, No. 1, 2011, pp. 9- 17. doi:10.1111/j.1524-4741.2010.01018.x
- [66] S. A. Jansen, “Ductal Carcinoma in Situ: Detection, Diagnosis, and Characterization with Magnetic Resonance Imaging,” Seminars in Ultrasound, CT and MR, Vol. 32, No. 4, 2011, pp. 306-318. doi:10.1053/j.sult.2011.02.007
- [67] R. J. Bleicher, et al., “Association of Routine Pretreatment Magnetic Resonance Imaging with Time to Surgery, Mastectomy Rate, and Margin Status,” Journal of the American College of Surgeons, Vol. 209, No. 2, 2009, pp. 180-187. doi:10.1016/j.jamcollsurg.2009.04.010
- [68] L. Turnbull, et al., “Comparative Effectiveness of MRI in Breast Cancer (COMICE) Trial: A Randomised Controlled Trial,” The Lancet, Vol. 375, No. 9714, 2010, pp. 563-571. doi:10.1016/S0140-6736(09)62070-5
- [69] L. W. Turnbull, et al., “Multicentre Randomised Controlled Trial Examining the Cost-Effectiveness of Contrast-Enhanced High Field Magnetic Resonance Imaging in Women with Primary Breast Cancer Scheduled for Wide Local Excision (COMICE),” Health Technology Assessment, Vol. 14, No. 1, 2010, pp. 1-182.
- [70] L. J. Solin, et al., “Relationship of Breast Magnetic Resonance Imaging to Outcome after Breast-Conservation Treatment with Radiation for Women with Early-Stage Invasive Breast Carcinoma or Ductal Carcinoma in Situ,” Journal of Clinical Oncology, Vol. 26, No. 3, 2008, pp. 386-391. doi:10.1200/JCO.2006.09.5448
- [71] M. Pilewskie, et al., “Effect of MRI on the Management of Ductal Carcinoma in Situ of the Breast,” Annals of Surgical Oncology, Vol. 20, No. 5, 2013, pp. 1522-1529. doi:10.1245/s10434-012-2771-y
- [72] C. M. Quinn and J. L. Ostrowski, “Cytological and Architectural Heterogeneity in Ductal Carcinoma in Situ of the Breast,” Journal of Clinical Pathology, Vol. 50, No. 7, 1997, pp. 596-599. doi:10.1136/jcp.50.7.596
- [73] B. S. Shoker and J. P. Sloane, “DCIS Grading Schemes and Clinical Implications,” Histopathology, Vol. 35, No. 5, 1999, pp. 393-400. doi:10.1046/j.1365-2559.1999.035005393.x
- [74] M. J. Silverstein, et al., “Prognostic Classification of Breast Ductal Carcinoma-in-Situ,” The Lancet, Vol. 345, No. 8958, 1995, pp. 1154-1157. doi:10.1016/S0140-6736(95)90982-6
- [75] M. J. Bagnall, et al., “Predicting Invasion in Mammographically Detected Microcalcification,” Clinical Radiology, Vol. 56, No. 10, 2001, pp. 828-832. doi:10.1053/crad.2001.0779
- [76] S. E. Pinder, et al., “A New Pathological System for Grading DCIS with Improved Prediction of Local Recurrence: Results from the UKCCCR/ANZ DCIS Trial,” British Journal of Cancer, Vol. 103, No. 1, 2010, pp. 94- 100. doi:10.1038/sj.bjc.6605718
- [77] S. E. Pinder, “Ductal Carcinoma in Situ (DCIS): Pathological Features, Differential Diagnosis, Prognostic Factors and Specimen Evaluation,” Modern Pathology, Vol. 23, Suppl. 2, 2010, pp. S8-S13. doi:10.1038/modpathol.2010.40
- [78] K. Polyak, I. Haviv and I. G. Campbell, “Co-Evolution of Tumor Cells and Their Microenvironment,” Trends in Genetics, Vol. 25, No. 1, 2009, pp. 30-38. doi:10.1016/j.tig.2008.10.012
- [79] D. C. Sgroi, “Preinvasive Breast Cancer,” Annual Review of Pathology, Vol. 5, 2010, pp. 193-221. doi:10.1146/annurev.pathol.4.110807.092306
- [80] E. S. Knudsen, et al., “Retinoblastoma and Phosphate and Tensin Homolog Tumor Suppressors: Impact on Ductal Carcinoma in Situ Progression,” Journal of the National Cancer Institute, Vol. 104, No. 23, 2013, pp. 1825-1836. doi:10.1093/jnci/djs446
- [81] P. R. Pandey, et al., “Elevated Lipogenesis in Epithelial Stem-Like Cell Confers Survival Advantage in Ductal Carcinoma in Situ of Breast Cancer,” Oncogene, 2012.
- [82] H. Buerger, et al., “Comparative Genomic Hybridization of Ductal Carcinoma in Situ of the Breast-Evidence of Multiple Genetic Pathways,” The Journal of Pathology, Vol. 187, No. 4, 1999, pp. 396-402. doi:10.1002/(SICI)1096-9896(199903)187:4<396::AID-PATH286>3.0.CO;2-L
- [83] H. Buerger, et al., “Different Genetic Pathways in the Evolution of Invasive Breast Cancer Are Associated with Distinct Morphological Subtypes,” The Journal of Pathology, Vol. 189, No. 4, 1999, pp. 521-526. doi:10.1002/(SICI)1096-9896(199912)189:4<521::AID-PATH472>3.0.CO;2-B
- [84] H. Buerger, et al., “Ductal Invasive G2 and G3 Carcinomas of the Breast Are the End Stages of at Least Two Different Lines of Genetic Evolution,” The Journal of Pathology, Vol. 194, No. 2, 2001, pp. 165-170. doi:10.1002/path.875
- [85] M. L. Gauthier, et al., “Abrogated Response to Cellular Stress Identifies DCIS Associated with Subsequent Tumor Events and Defines Basal-Like Breast Tumors,” Cancer Cell, Vol. 12, No. 5, 2007, pp. 479-491. doi:10.1016/j.ccr.2007.10.017
- [86] J. Lu, et al., “14-3-3zeta Cooperates with ErbB2 to Promote Ductal Carcinoma in Situ Progression to Invasive Breast Cancer by Inducing Epithelial-Mesenchymal Transition,” Cancer Cell, Vol. 16, No. 3, 2009, pp. 195-207. doi:10.1016/j.ccr.2009.08.010
- [87] L. Qi, et al., “Expression of miR-21 and Its Targets (PTEN, PDCD4, TM1) in Flat Epithelial Atypia of the Breast in Relation to Ductal Carcinoma in Situ and Invasive Carcinoma,” BMC Cancer, Vol. 9, 2009, p. 163. doi:10.1186/1471-2407-9-163
- [88] L. F. Sempere, et al., “Altered MicroRNA Expression Confined to Specific Epithelial Cell Subpopulations in Breast Cancer,” Cancer Research, Vol. 67, No. 24, 2007, pp. 11612-11620. doi:10.1158/0008-5472.CAN-07-5019
- [89] X. Catteau, et al., “Variable Stromal Periductular Expression of CD34 and Smooth Muscle Actin (SMA) in Intraductal Carcinoma of the Breast,” PLoS One, Vol. 8, No. 3, 2013, p. e57773. doi:10.1371/journal.pone.0057773
- [90] L. J. Solin, et al., “A Multigene Expression Assay to Predict Local Recurrence Risk for Ductal Carcinoma in Situ of the Breast,” Journal of the National Cancer Institute, Vol. 105, No. 10, 2013, pp. 701-710. doi:10.1093/jnci/djt067
- [91] S. Y. Wang, et al., “Network Meta-Analysis of Margin Threshold for Women with Ductal Carcinoma in Situ,” Journal of the National Cancer Institute, Vol. 104, No. 7, 2012, pp. 507-516. doi:10.1093/jnci/djs142
- [92] C. Dunne, et al., “Effect of Margin Status on Local Recurrence after Breast Conservation and Radiation Therapy for Ductal Carcinoma in Situ,” Journal of Clinical Oncology, Vol. 27, No. 10, 2009, pp. 1615-1620. doi:10.1200/JCO.2008.17.5182
- [93] M. J. Silverstein, et al., “A Prognostic Index for Ductal Carcinoma in Situ of the Breast,” Cancer, Vol. 77, No. 11, 1996, pp. 2267-2274. doi:10.1002/(SICI)1097-0142(19960601)77:11<2267::AID-CNCR13>3.0.CO;2-V
- [94] M. J. Silverstein and M. D. Lagios, “Choosing Treatment for Patients with Ductal Carcinoma in Situ: Fine Tuning the University of Southern California/Van Nuys Prognostic Index,” Journal of the National Cancer Institute. Monographs, Vol. 2010, No. 41, 2010, pp. 193-196. doi:10.1093/jncimonographs/lgq040
- [95] L. Nekhlyudov, et al., “Ten-Year Risk of Diagnostic Mammograms and Invasive Breast Procedures after Breast-Conserving Surgery for DCIS,” Journal of the National Cancer Institute, Vol. 104, No. 8, 2012, pp. 614- 621. doi:10.1093/jnci/djs167
- [96] D. E. Fisher, et al., “Chest Wall Recurrence of Ductal Carcinoma in Situ of the Breast after Mastectomy,” Cancer, Vol. 71, No. 10, 1993, pp. 3025-3028. doi:10.1002/1097-0142(19930515)71:10<3025::AID-CNCR2820711023>3.0.CO;2-Z
- [97] B. Gerber, et al., “The Oncological Safety of Skin Sparing Mastectomy with Conservation of the Nipple-Areola Complex and Autologous Reconstruction: An Extended Follow-Up Study,” Annals of Surgery, Vol. 249, No. 3, 2009, pp. 461-468. doi:10.1097/SLA.0b013e31819a044f
- [98] E. F. Brachtel, et al., “Occult Nipple Involvement in Breast Cancer: Clinicopathologic Findings in 316 Consecutive Mastectomy Specimens,” Journal of Clinical Oncology, Vol. 27, No. 30, 2009, pp. 4948-4954. doi:10.1200/JCO.2008.20.8785
- [99] L. A. Lee, et al., “Breast Cancer-Specific Mortality after Invasive Local Recurrence in Patients with Ductal Carcinoma-in-Situ of the Breast,” The American Journal of Surgery, Vol. 192, No. 4, 2006, pp. 416-419. doi:10.1016/j.amjsurg.2006.06.005
- [100] A. P. Schouten van der Velden, et al., “Local Recurrences after Different Treatment Strategies for Ductal Carcinoma in Situ of the Breast: A Population-Based Study in the East Netherlands,” International Journal of Radiation Oncology, Biology, Physics, Vol. 69, No. 3, 2007, pp. 703-710. doi:10.1016/j.ijrobp.2007.03.062
- [101] A. Rashtian, et al., “Close or Positive Margins after Mastectomy for DCIS: Pattern of Relapse and Potential Indications for Radiotherapy,” International Journal of Radiation Oncology, Biology, Physics, Vol. 72, No. 4, 2008, pp. 1016-1020. doi:10.1016/j.ijrobp.2008.06.1954
- [102] J. H. Kim, F. Tavassoli and B. G. Haffty, “Chest Wall Relapse after Mastectomy for Ductal Carcinoma in Situ: A Report of 10 Cases with a Review of the Literature,” Cancer Journal, Vol. 12, No. 2, 2006, pp. 92-101.
- [103] E. R. Fisher, et al., “Conservative Management of Intraductal Carcinoma (DCIS) of the Breast. Collaborating NSABP Investigators,” Journal of Surgical Oncology, Vol. 47, No. 3, 1991, pp. 139-147. doi:10.1002/jso.2930470302
- [104] I. Naoura, et al., “Factors Influencing the Decision to Offer Immediate Breast Reconstruction after Mastectomy for Ductal Carcinoma in Situ (DCIS): The Institut Gustave Roussy Breast Cancer Study Group Experience,” Breast, 2013, Article ID: pii: S0960-9776(13)00004-0.
- [105] M. J. Silverstein, et al., “Axillary Lymph Node Dissection for Intraductal Breast Carcinoma—Is It Indicated?” Cancer, Vol. 59, No. 10, 1987, pp. 1819-1824. doi:10.1002/1097-0142(19870515)59:10<1819::AID-CNCR2820591023>3.0.CO;2-V
- [106] T. B. Julian, et al., “Is Sentinel Node Biopsy Necessary in Conservatively Treated DCIS?” Annals of Surgical Oncology, Vol. 14, No. 8, 2007, pp. 2202-2208. doi:10.1245/s10434-007-9353-4
- [107] V. Trisal, D. Qian and L. D. Wagman, “Axillary Recurrence in DCIs: Is Axillary Lymphadenectomy Warranted?” The American Surgeon, Vol. 70, No. 10, 2004, pp. 876-880.
- [108] R. J. Jackman, et al., “Stereotactic, Automated, LargeCore Needle Biopsy of Nonpalpable Breast Lesions: False-Negative and Histologic Underestimation Rates after Long-Term Follow-Up,” Radiology, Vol. 210, No. 3, 1999, pp. 799-805.
- [109] C. H. Lee, et al., “Ductal Carcinoma in Situ Diagnosed with Stereotactic Core Needle Biopsy: Can Invasion Be Predicted?” Radiology, Vol. 217, No. 2, 2000, pp. 466- 470.
- [110] S. Pandelidis, et al., “Accuracy of 11-Gauge VacuumAssisted Core Biopsy of Mammographic Breast Lesions,” Annals of Surgical Oncology, Vol. 10, No. 1, 2003, pp. 43-47. doi:10.1245/ASO.2003.05.004
- [111] R. J. Jackman, et al., “Stereotactic Breast Biopsy of Nonpalpable Lesions: Determinants of Ductal Carcinoma in Situ Underestimation Rates,” Radiology, Vol. 218, No. 2, 2001, pp. 497-502.
- [112] S. Pendas, et al., “Sentinel Node Biopsy in Ductal Carcinoma in Situ Patients,” Annals of Surgical Oncology, Vol. 7, No. 1, 2000, pp. 15-20. doi:10.1007/s10434-000-0015-z
- [113] N. Klauber-DeMore, et al., “Sentinel Lymph Node Biopsy: Is It Indicated in Patients with High-Risk Ductal Carcinoma-in-Situ and Ductal Carcinoma-in-Situ with Microinvasion?” Annals of Surgical Oncology, Vol. 7, No. 9, 2000, pp. 636-642. doi:10.1007/s10434-000-0636-2
- [114] C. E. Cox, et al., “Importance of Lymphatic Mapping in Ductal Carcinoma in Situ (DCIS): Why Map DCIS?” The American Surgeon, Vol. 67, No. 6, 2001, pp. 513-519.
- [115] T. W. Yen, et al., “Predictors of Invasive Breast Cancer in Patients with an Initial Diagnosis of Ductal Carcinoma in Situ: A Guide to Selective Use of Sentinel Lymph Node Biopsy in Management of Ductal Carcinoma in Situ,” Journal of the American College of Surgeons, Vol. 200, No. 4, 2005, pp. 516-526. doi:10.1016/j.jamcollsurg.2004.11.012
- [116] M. J. Silverstein, et al., “Intraductal Carcinoma of the Breast (208 cases). Clinical Factors Influencing Treatment Choice,” Cancer, Vol. 66, No. 1, 1990, pp. 102-108. doi:10.1002/1097-0142(19900701)66:1<102::AID-CNCR2820660119>3.0.CO;2-5
- [117] M. D. Lagios, et al., “Duct Carcinoma in Situ. Relationship of Extent of Noninvasive Disease to the Frequency of Occult Invasion, Multicentricity, Lymph Node Metastases, and Short-Term Treatment Failures,” Cancer, Vol. 50, No. 7, 1982, pp. 1309-1314.
- [118] H. Mabry, A. E. Giuliano and M. J. Silverstein, “What Is the Value of Axillary Dissection or Sentinel Node Biopsy in Patients with Ductal Carcinoma in Situ?” The American Journal of Surgery, Vol. 192, No. 4, 2006, pp. 455- 457. doi:10.1016/j.amjsurg.2006.06.028
- [119] M. Intra, et al., “Sentinel Node Biopsy Is Not a Standard Procedure in Ductal Carcinoma in Situ of the Breast: The Experience of the European Institute of Oncology on 854 Patients in 10 Years,” Annals of Surgery, Vol. 247, No. 2, 2008, pp. 315-319. doi:10.1097/SLA.0b013e31815b446b
- [120] C. D. Murphy, et al., “Do Sentinel Node Micrometastases Predict Recurrence Risk in Ductal Carcinoma in Situ and Ductal Carcinoma in Situ with Microinvasion? The American Journal of Surgery, Vol. 196, No. 4, 2008, pp. 566-568. doi:10.1016/j.amjsurg.2008.06.011
- [121] J. F. Lara, et al., “The Relevance of Occult Axillary Micrometastasis in Ductal Carcinoma in Situ: A Clinicopathologic Study with Long-Term Follow-Up,” Cancer, Vol. 98, No. 10, 2003, pp. 2105-2113. doi:10.1002/cncr.11761
- [122] J. C. Tan, et al., “Role of Sentinel Lymph Node Biopsy in Ductal Carcinoma-in-Situ Treated by Mastectomy,” Annals of Surgical Oncology, Vol. 14, No. 2, 2007, pp. 638- 645. doi:10.1245/s10434-006-9211-9
- [123] M. Intra, et al., “Sentinel Lymph Node Biopsy Is Feasible even after Total Mastectomy,” Journal of Surgical Oncology, Vol. 95, No. 2, 2007, pp. 175-179. doi:10.1002/jso.20670
- [124] B. Fisher, et al., “Lumpectomy and Radiation Therapy for the Treatment of Intraductal Breast Cancer: Findings from National Surgical Adjuvant Breast and Bowel Project B-17,” Journal of Clinical Oncology, Vol. 16, No. 2, 1998, pp. 441-452.
- [125] E. R. Fisher, et al., “Pathologic Findings from the National Surgical Adjuvant Breast Project (NSABP) EightYear Update of Protocol B-17: Intraductal Carcinoma,” Cancer, Vol. 86, No. 3, 1999, pp. 429-438. doi:10.1002/(SICI)1097-0142(19990801)86:3<429::AID-CNCR11>3.0.CO;2-Y
- [126] B. Fisher, et al., “Prevention of Invasive Breast Cancer in Women with Ductal Carcinoma in Situ: An Update of the National Surgical Adjuvant Breast and Bowel Project Experience,” Seminars in Oncology, Vol. 28, No. 4, 2001, pp. 400-418. doi:10.1016/S0093-7754(01)90133-2
- [127] I. L. Wapnir, et al., “Long-Term Outcomes of Invasive Ipsilateral Breast Tumor Recurrences after Lumpectomy in NSABP B-17 and B-24 Randomized Clinical Trials for DCIS,” Journal of the National Cancer Institute, Vol. 103, No. 6, 2011, pp. 478-488. doi:10.1093/jnci/djr027
- [128] N. Bijker, et al., “Breast-Conserving Treatment with or without Radiotherapy in Ductal Carcinoma-in-Situ: Ten-Year Results of European Organisation for Research and Treatment of Cancer Randomized Phase III Trial 10853—A Study by the EORTC Breast Cancer Cooperative Group and EORTC Radiotherapy Group,” Journal of Clinical Oncology, Vol. 24, No. 21, 2006, pp. 3381-3387. doi:10.1200/JCO.2006.06.1366
- [129] N. Bijker, et al., “Risk Factors for Recurrence and Metastasis after Breast-Conserving Therapy for Ductal Carcinoma-in-Situ: Analysis of European Organization for Research and Treatment of Cancer Trial 10853,” Journal of Clinical Oncology, Vol. 19, No. 8, 2001, pp. 2263- 2271.
- [130] L. Holmberg, et al., “Absolute Risk Reductions for Local Recurrence after Postoperative Radiotherapy after Sector Resection for Ductal Carcinoma in Situ of the Breast,” Journal of Clinical Oncology, Vol. 26, No. 8, 2008, pp. 1247-1452. doi:10.1200/JCO.2007.12.7969
- [131] J. Houghton, et al., “Radiotherapy and Tamoxifen in Women with Completely Excised Ductal Carcinoma in Situ of the Breast in the UK, Australia, and New Zealand: Randomised Controlled Trial,” The Lancet, Vol. 362, No. 9378, 2003, pp. 95-102. doi:10.1016/S0140-6736(03)13859-7
- [132] NIH State of the Science Conference, “Diagnosis and Management of Ductal Carcinoma in Situ (DCIS),” Bethesda, 22-24 September 2009. http://consensus.nih.gov/2009/dcis.htm
- [133] M. Clarke, et al., “Effects of Radiotherapy and of Differences in the Extent of Surgery for Early Breast Cancer on Local Recurrence and 15-Year Survival: An Overview of the Randomised Trials,” The Lancet, Vol. 366, No. 9503, 2005, pp. 2087-2106.
- [134] L. J. Solin, et al., “Long-Term Outcome after BreastConservation Treatment with Radiation for Mammographically Detected Ductal Carcinoma in Situ of the Breast,” Cancer, Vol. 103, No. 6, 2005, pp. 1137-1146. doi:10.1002/cncr.20886
- [135] E. Rakovitch, et al., “Can We Select Individuals with Low Risk Ductal Carcinoma in Situ (DCIS)? A Population-Based Outcomes Analysis,” Breast Cancer Research and Treatment, Vol. 138, No. 2, 2013, pp. 581-590. doi:10.1007/s10549-013-2455-8
- [136] L. L. Hughes, et al., “Local Excision alone without Irradiation for Ductal Carcinoma in Situ of the Breast: A Trial of the Eastern Cooperative Oncology Group,” Journal of Clinical Oncology, Vol. 27, No. 32, 2009, pp. 5319-5324. doi:10.1200/JCO.2009.21.8560
- [137] R. L. Balleine, et al., “Molecular Grading of Ductal Carcinoma in Situ of the Breast,” Clinical Cancer Research, Vol. 14, No. 24, 2008, pp. 8244-8252. doi:10.1158/1078-0432.CCR-08-0939
- [138] B. Fisher, et al., “Tamoxifen in Treatment of Intraductal Breast Cancer: National Surgical Adjuvant Breast and Bowel Project B-24 Randomised Controlled Trial,” The Lancet, Vol. 353, No. 9169, 1999, pp. 1993-2000. doi:10.1016/S0140-6736(99)05036-9
- [139] B. Fisher, et al., “Tamoxifen for the Prevention of Breast Cancer: Current Status of the National Surgical Adjuvant Breast and Bowel Project P-1 Study,” Journal of the National Cancer Institute, Vol. 97, No. 22, 2005, pp. 1652- 1662. doi:10.1093/jnci/dji372
- [140] J. M. Dixon, et al., “DCIS and Aromatase Inhibitors,” The Journal of Steroid Biochemistry and Molecular Biology, Vol. 106, No. 1-5, 2007, pp. 173-179.
- [141] Y. Y. Chen, et al., “Pathologic and Biologic Response to Preoperative Endocrine Therapy in Patients with ER-Positive Ductal Carcinoma in Situ,” BMC Cancer, Vol. 9, 2009, p. 285. doi:10.1186/1471-2407-9-285
- [142] N. J. Bundred, et al., “Cyclooxygenase-2 Inhibition Does Not Improve the Reduction in Ductal Carcinoma in Situ Proliferation with Aromatase Inhibitor Therapy: Results of the ERISAC Randomized Placebo-Controlled Trial,” Clinical Cancer Research, Vol. 16, No. 5, 2010, pp. 1605-1612. doi:10.1158/1078-0432.CCR-09-1623
- [143] D. C. Allred, et al., “Overexpression of HER-2/neu and Its Relationship with Other Prognostic Factors Change during the Progression of in Situ to Invasive Breast Cancer,” Human Pathology, Vol. 23, No. 9, 1992, pp. 974- 979. doi:10.1016/0046-8177(92)90257-4
- [144] G. Farnie, et al., “Combined Inhibition of ErbB1/2 and Notch Receptors Effectively Targets Breast Ductal Carcinoma in Situ (DCIS) Stem/Progenitor Cell Activity Regardless of ErbB2 Status,” PLoS One, Vol. 8, No. 2, 2013, p. e56840. doi:10.1371/journal.pone.0056840
- [145] E. Half, et al., “Cyclooxygenase-2 Expression in Human Breast Cancers and Adjacent Ductal Carcinoma in Situ,” Cancer Research, Vol. 62, No. 6, 2002, pp. 1676-1681.
- [146] S. C. Lester, et al., “Protocol for the Examination of Specimens from Patients with Ductal Carcinoma in Situ of the Breast,” Archives of Pathology & Laboratory Medicine, Vol. 133, No. 1, 2009, pp. 15-25.
- [147] J. P. Julien, et al., “Radiotherapy in Breast-Conserving Treatment for Ductal Carcinoma in Situ: First Results of the EORTC Randomised Phase III Trial 10853. EORTC Breast Cancer Cooperative Group and EORTC Radiotherapy Group,” The Lancet, Vol. 355, No. 9203, 2000, pp. 528-533. doi:10.1016/S0140-6736(99)06341-2
- [148] Clinical Trial, “Radiation Therapy with or without Trastuzumab in Treating Women with Ductal Carcinoma in Situ Who Have Undergone Lumpectomy,” National Institutes of Health, Bethesda, 2009. http://clinicaltrials.gov/ct2/show/NCT00769379
- [149] H. M. Kuerer, et al., “Biologic and Immunologic Effects of Preoperative Trastuzumab for Ductal Carcinoma in Situ of the Breast,” Cancer, Vol. 117, No. 1, 2011, pp. 39-47. doi:10.1002/cncr.25399
- [150] Clinical Trial, “Lapatinib in Treating Women with Ductal Carcinoma in Situ of the Breast,” National Institutes of Health, Bethesda, 2009 http://clinicaltrials.gov/ct2/show/NCT00570453
NOTES
*Corresponding author.

