3-(Tetrazol-5-yl)-2-imino-coumarins Derivatives: Synthesis, Characterization, and Evaluation on Tumor Cell Lines ()
1. Introduction
Tetrazole [1] belongs to a family of compounds bearing the highest number of nitrogen atoms and surprisingly, they do not exist in nature. Tetrazole is an important scaffold because they are integrated in many compounds that have applications in numerous fields such as in medicine, biochemistry, and pharmacology [2] [3] [4]. It’s noteworthy that in pharmacology, 5-substituted-1H-tetrazoles are bio-isosters of carboxylic acids because they presented comparable pKa (tetrazole 4.5 - 4.9 vs carboxylic acid 4.2 - 4.4), they have a similar size and a near molecular electrostatic potential [5]. They undergo very similar receptor-ligand interactions [6], exhibit a prolonged half-life time because they enhanced the metabolic stability [7] [8] and the membrane penetration [9]. Food Drug Administration (FDA) approved 23 drugs that contain 1H- or 2H-tetrazole substituents [10]. Among them as examples (Figure 1): losartan as angiotensin II receptor [11], irbesartan as Angiotensin Receptor Blocker (ARB) for the treatment of hypertension [12], cilostazol for peripheral vascular disease [13] and cefazolin as antibiotic [14].
In our group and as part of our program aimed at developing new methods for the preparation of new building blocks or, for the synthesis of 2-iminocoumarins showing potential biological properties dedicated to protection of rat tissues from isoproterenol toxicity [15] or, for cancer [16] [17], we were motivated in this work respectively for: 1) to prepare a class of hybrid derivatives of 2-iminocoumarins bearing a tetrazole pharmacophore, 2) to evaluate their antiproliferative activities on tumor cell lines and iii) to study their UV/visible properties.
2. Results and Discussion
2.1. Synthesis and Characterizations
The synthetic route towards the preparation of the title compounds 5 is outlined below. In the first step (Scheme 1), the 3-cyano-2-iminocoumarins 3 were synthesized from an equimolecular mixture of various substituted 2-hydroxy benzaldehyde 1 (1a: 2-hydroxy-3-methoxybenzaldehyde, 1b: 4-diethylamino-2-hydroxybenzaldehyde, 1c: 2,4-dihydroxybenzaldehyde, 1d: 2-hydroxynaphthaldehyde, 1e: 2-hydroxybenzaldehyde, 1f: 2-hydroxy-4-methoxybnezaldehyde, 1g: 5-bromo-2-hydroxybenzaldehyde, 1h: 3-ethoxy-2-hydroxybenzaldehyde) and
![]()
Figure 1. Selected drugs approved by FDA containing the tetrazole moiety.
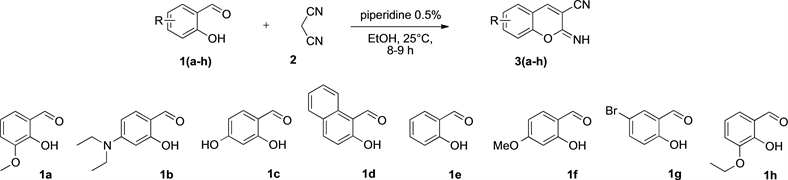
Scheme 1. Synthesis of 3-cyano-2-iminocoumarins 3(a-h) from various substituted 2-hydroxybenzaldehydes 1(a-h) and propanedinitrile 2.
propanedinitrile 2 in ethanol with 0.5% of piperidine at room temperature. After a reaction time of 8 - 9 hours and elimination of volatile compounds in vacuo, the 3-cyano-2-iminocoumarins 3 were prepared easily according to this classical protocol previously developed in our laboratory [18] [19] [20].
In the second step (Scheme 2), transformation of the nitrile group of 3-cyano2-iminocoumarins 3 into desired tetrazole moiety [21] was accomplished by using one equivalent of zinc chloride as catalyst and sodium azide 4. The reaction was conducted in a solution of THF and deionized water (4:1 v/v) under reflux during 4 - 7 h. Completion of the reaction was monitored by thin layer chromatography on 0.2-mm precoated plates of silica gel 60F-254 (Merck). After cooling down to room temperature, the precipitated material was collected by filtration on a Buchner funnel (porosity N˚4) and purified by washing with deionized water. The desired 3-(tetrazol-5-yl)-2-iminocoumarins 5 were synthesized in yields ranging from 55 to 92 (Table 1). Moderate yields must be mentioned for compounds 5c (61%) and 5g (55%) bearing respectively a phenolic function in C-7 position for 5c and a bromine atom in C-6 position for 5g. On the contrary, the presence of electron-donating groups, such as methoxy or ethoxy groups provide yields greater than 90%, this concerns 5a (92%) and 5h (93%).
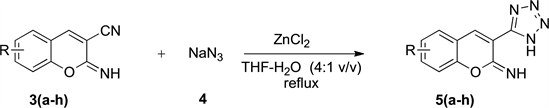
Scheme 2. Synthesis of substituted 3-(tetrazol-5-yl)-2-iminocoumarins 5(a-h) from various substituted 3-cyano-2-iminocoumarins 3(a-h) and sodium azide 4.
![]()
Table 1. Results for the preparation of 3-(tetrazol-5-yl)-2-iminocoumarins 5(a-h).
aIsolated yields.
The structures for hybrid derivatives of 2-iminocoumarins 5(a-h) were confirmed by 1H, 13C NMR, HRMS and FTIR. In the IR spectrum, the presence of NH stretching frequencies of the tetrazole group of 5 was detected at 3400 cm−1 [22] and associated to disappearance of the characteristic band at 2200 - 2300 cm−1 for CN group of the 3-cyano-2-iminocoumarins 3. This is also confirmed in 13C NMR by the disappearance of a peak located at δ115 (attributed to the CN group of the starting compound 3) after synthesis of compounds 5. For MS, the [M + 1]+ molecular ion signal for all compounds 5 were obtained as base signal.
UV/Visible experiments were realized in DMSO and analytical data are reported in Table 2. Examination of these results shows that absorption maxima λabs of compounds 5 are located between 320 to 444 nm. Their emission peaks appears in purple-blue region due to a large Stokes shift (see Figure 2 for compound 5b in DMSO) and their fluorescence excitation spectra were similar. Highest values of quantum yields f were observed respectively for compounds 5c (f = 0.68) and moderate values for compounds 5b (f = 0.362), 5f (f = 0.335)
![]()
Table 2. Maximum absorption (λabs) and emission (λem) wavelengths, and fluorescence quantum yields for the 3-(tetrazol-5-yl)-2-iminocoumarins 5(a-h).
![]()
Figure 2. Normalized absorption (bold line) and emission (dotted line) spectrum of 2-diethylamino-3-(tetrazol-5yl)-2-iminocoumarin 5b in DMSO.
bearing a substituent in C-7 position. The presence of a donor group leaded to potential interesting quantum yields.
2.2. Cytotoxic Assays
The potential in vitro cytotoxic character of 3-(tetrazol-5-yl)-2-iminocoumarins 5(a-h) has been evaluated on six selected tumor cell lines, which are respectively Huh7-D12, Caco 2, MDA-MB231, HCT 116, PC3 and NCI-H727 and are representative of different cancers (leukemia, melanoma and cancers for liver, colon, breast, prostate, lung and kidney). HaCat keratinocyte was also used as normal cell line. For each tumor cell line, the % of cell survival was measured at a single dose of 25 μM (after 48 h) in triplicate. When the survival rate is less than 50%, the IC50 value for this compound is determined, using roscovitine and doxorubicine as references for positive controls (Table 3). It can be observed that only compound 5e exhibited antiproliferative activity with a marked effect on HCT116 (5e: IC50 15 μM).
3. Conclusion
This work described an easy method for the synthesis of new 3-(tetrazol-5-yl)-2-imino-coumarins derivatives. Introduction of the tetrazol moiety on the 3-cyano function of 2-iminocoumarins 3(a-h) involves a very simple method by using zinc chloride as catalyst that provided the desired compounds in yields ranging from 55% to 92%. UV/Visible analytical data were also realized by measuring the absorption maxima λabs, the emission maxima λem associated to fluorescence quantum yields f. Antiproliferative activities of all the
![]()
Table 3. Antiproliferative activities of 3-(tetrazol-5-yl)-2-iminocoumarins 5(a-h) on six tumor cell lines and HaCat keratinocyte.
aPercentage of survival measured at 25 μM (after 48 h using a single dose, triplicate). bIC50 values in parentheses are expressed in μM and are the average of three assays, standard error ± 0.5 μM.
new compounds were also examined on six representative human tumor cell lines and we observed that compound 5e was active on HCT 116 (IC50 15 μM). These current results are the starting point of a new larger program within our group to investigate intensively the biological properties of these new compounds with potential applications in cancer.
4. Experimental Sections
4.1. Chemistry
4.1.1. General Information
Solvents were evaporated with a BUCHI rotary evaporator (New Castle, PA, USA). All reagents and solvents were purchased from Acros Fisher (Illkirch, France), Sigma-Aldrich Chimie (St Quentin Fallavier, France) and were used without further purification. 1H NMR spectra were recorded on Bruker AC 300 P (300 MHz) spectrometer and 13C NMR spectra on Bruker AC 300 P (75 MHz) spectrometer (Bruker France Scientifique, Voisins-le-Bretonneux, France). Chemical shifts are expressed in parts per million downfield. Data are given in the following order: δvalue, multiplicity (s, singlet; d, doublet; t, triplet; q, quartet; quint: quintuplet, m, multiplet; br, broad), number of protons, coupling constants J is given in Hertz. The High-Resolution Mass Spectra (HRMS) were recorded in positive mode using direct Electrospray infusion, respectively on Waters Q-TOF 2 or on Thermo Fisher Scientific Q-Exactive spectrometers (Thermo Electron, Villebon-sur-Yvette, France) at the “Centre Régional de Mesures Physiques de l’Ouest” platform (CRMPO platform, ScanMAT UMS 2001 CNRS, Rennes, France). Melting points were determined on a Kofler melting point apparatus and were uncorrected. Infrared spectra were recorded on a Perkin Elmer 100 (Perkin Elmer France, Paris, France). UV/VIS absorption spectra were recorded on a Hewlett Packard 8452A diode array spectrophotometer (Hewlett Packard Enterprise, Paris, France). For solutions, corrected steady state fluorescence spectra were recorded with a Perkin Elmer LS55 spectrofluorometer using cells of 1 cm optical pathway (Perkin Elmer France, Paris, France). Elemental microanalyses were performed on an EA1112 analyzer from CE Instruments Hi Tech Detection Systems HTDS, ZI Charguia II 2035, Tunis-Carthage, Tunisie).
4.1.2. General Procedure for the Synthesis of 3-(tetrazol-5-yl)-2-iminocoumarins 5(a-h)
To a stirred solution of 2-iminocoumarin-3-carbonitrile 3 (0.27 mmol) in a solution of 4 mL of THF and 1 mL of deionized water, was added successively zinc chloride (37 mg, 0.32 mml) and sodium azide (20 mg, 0.32 mmol). The resulting mixture was refluxed under magnetic stirring (500 rpm) for a reaction time from 4 to 7 h. After cooling down to room temperature, the desired compound 5 was collected by filtration on a Büchner funnel (porosity N˚4), washed with cooled deionized water (2 × 2 mL), dried under vacuum (10 - 2 Torr) at 25˚C for 1 h to give a powder.
1)8-Methoxy-3-(tetrazol-5-yl)-2-iminocoumarin (5a)
Yield = 92%. Reaction time = 4 h. Mp > 260˚C. IR (cm−1): ν = 3295 (NH), 1634 (C=N); 1H NMR (300 MHz, CDCl3) δ: 11.21 (br s, 1H, NH), 9.32 (s, 1H, H4), 7.54 (m, 3H, HAr), 4.02 (s, 3H, OCH3); 13C NMR (75 MHz, CDCl3) δ: 147.5, 121.3, 120.9, 120.1, 119.2, 118,3 116.3, 112.5, 108.7, 56.6. Anal. Calcd for C11H9N5O2: C, 54.32; H, 3.73; N, 28.79. Found C, 54.26; H, 3.66; N, 28.71.
2)7-N,N-Diethylamino-3-(tetrazol-5-yl)-2-iminocoumarin (5b)
Yield = 87%. Reaction time = 4 h. Mp > 260˚C. IR (cm−1): ν = 3229 (NH), 1643 (C=N); 1H NMR (300 MHz, DMSO-d6) δ: 11.20 (br s, 1H, NH), 8.81 (s, 1H, H4), 7.69 (d, 3J = 8.7 Hz, 1H, H5), 6.82 (d, 3J = 8.7 Hz, 1H, H6), 6.56 (s, 1H, H8), 3.50 (q, 3J = 13.8 Hz, 2H, CH2), 1.17 (t, 3J = 13.8 Hz, 3H, CH3); 13C NMR (75 MHz, DMSO-d6) δ: 161.9, 155.8, 154.5, 151.4, 143.05, 130.3, 110.1, 107.9, 106.7, 95.61, 44.2, 12.2. Anal. Calcd for C14H16N6O: C, 59.14; H, 5.67; N, 29.56. Found C, 59.23; H, 5.61; N, 29.59.
3)7-Hydroxy-3-(tetrazol-5-yl)-2-iminocoumarin (5c)
Yield = 61%. Reaction time = 7 h. Mp > 260˚C. IR (cm−1): ν = 3321 (NH), 1646 (C=N); 1H NMR (300 MHz, DMSO-d6) δ: 8.97 (br s, 1H, NH), 8.89 (s, 1H, H4), 7.78 (d, 3J = 8.1 Hz, 1H, H5), 6.88 (d, 3J = 8.4 Hz, 1H, H6), 6.80 (s, 1H, H8); 13C NMR (75 MHz, DMSO-d6) δ: 163.1, 160.6, 155.7, 153.8, 139.4, 130.9, 114.4, 111.0, 110.8, 101.7. Anal. Calcd for C10H7N5O2: C, 52.40; H, 3.08; N, 30.56. Found C, 52.35; H, 3.05; N, 30.22.
4)2-(Tetrazol-5-yl)-3-imino-3H-naphtho[2,1-b]pyran (5d)
Yield = 73%. Reaction time = 4 h. Mp > 260˚C. IR (cm−1): ν = 3262 (NH), 1636 (C=N); 1H NMR (300 MHz, DMSO-d6) δ: 9.51 (br s, 1H, NH), 8.91 (s, 1H, H4), 8.72 (d, 3J = 9.0 Hz, 1H, H5’/6’), 8.36 (d, 3J = 9.0 Hz, 1H, H7), 8.13 (d, 3J = 9.0 Hz, 1H, H5’/6’), 7.81 (t, 3J = 6.1 Hz, 1H, H5”/6”), 7.72 (t, 3J = 3.0 Hz, 1H, H5”/6”); 6.76 (d,3J = 9.0 Hz, 1H, H8), 13C NMR (75 MHz, DMSO-d6) δ: 154.0, 149.7, 139.9, 135.4, 129.9, 128.9, 126.5, 122.3, 120.9, 116.4, 113.3, 112.6, 111.2, 109.4. Anal. Calcd for C14H9N5O: C, 63.87; H, 3.45; N, 26.61. Found C, 63.95; H, 3.41; N, 26.54.
5)3-(Tetrazol-5-yl)-2-iminocoumarin (5e)
Yield = 72%. Reaction time = 4 h. Mp > 260˚C. IR (cm−1): ν = 3313 (NH), 1641 (C=N); 1H NMR (300 MHz, DMSO-d6) δ: 8.55 (br s, 1H, NH), 8.27 (s, 1H, H4), 8.02 (dd, 1H, H8), 7.76 (m, 1H, H6), 7.55 (dd, 1H, H5), 7.48 (m, 1H, H7); 13C NMR (75 MHz, DMSO-d6) δ: 153.5, 149.5, 144.2, 133.7, 129.8, 125.0, 120.8, 118.3, 112.3, 109.3. Anal. Calcd for C10H7N5O: C, 56.34; H, 3.31; N, 32.85. Found C, 56.37; H, 3.29; N, 32.88.
6)7-Methoxy-3-(tetrazol-5-yl)-2-iminocoumarin (5f)
Yield = 84%. Reaction time = 6 h. Mp > 260˚C. IR (cm−1): ν = 3311 (NH), 1640 (C=N); 1H NMR (300 MHz, DMSO-d6) δ: 8.99 (br s, 1H, NH), 8.52 (s, 1H, H4), 7.93 (d, 3J = 9.0 Hz, 1H, H5), 7.05 (d, 3J = 6.0 Hz, 1H, H6), 6.99 (s, 1H, H8), 3.92 (s, 1H, OCH3); 13C NMR (75 MHz, DMSO-d6) δ: 164.3, 155.8, 149.7, 144.5, 131.15, 121.4, 111.9, 109.8, 108.2, 100.5, 56.2. Anal. Calcd for C11H9N5O2: C, 54.32; H, 3.73; N, 28.79. Found C, 54.41; H, 7.67; N, 28.82.
7)6,8-Dibromo-3-(tetrazol-5-yl)-2-iminocoumarin (5g)
Yield = 55%. Reaction time = 6 h. Mp > 260˚C. IR (cm−1): ν = 3205 (NH), 1651 (C=N); 1H NMR (300 MHz, DMSO-d6) δ: 9.46 (br s, 1H, NH), 8.62 (s, 1H, H4), 8.22 (s, 1H, H7), 8.09 (s, 1H, H5); 13C NMR (75 MHz, DMSO-d6) δ: 160.4, 150, 146.4, 136.3, 135.3, 130.3, 129.6, 120.9, 115.9, 107.6. Anal. Calcd for C10H5N5OBr2: C, 32.34; H, 1.35; N, 18.87. Found C, 32.39; H, 1.30; N, 18.92.
8)8-Ethoxy-3-(tetrazol-5-yl)-2-iminocoumarin (5h)
Yield = 93%. Reaction time = 6 h. Mp > 260˚C. IR (cm−1): ν = 3305 (NH), 1636 (C=N); 1H NMR (300 MHz, DMSO-d6) δ: 8.85 (br s, 1H, NH), 8.23 (s, 1H, H4), 7.35 - 7.43 (m, 3H, HAr), 4.23 (q, 3J = 12.0 Hz, 2H, OCH2), 1.43 (t, 3J = 12.0 Hz, 3H, CH3); 13C NMR (75 MHz, DMSO-d6) δ: 145.6, 144.5, 142.9, 125.1, 121.0, 120.8, 119.0, 116.6, 112.4, 109.5, 64.4, 14.4. Anal. Calcd for C12H11N5O2: C, 56.03; H, 4.31; N, 27.22. Found C, 55.98; H, 4.29; N, 27.29.
4.2. Cell Culture and Survival Assays
Caco2 (differentiated colorectal adenocarcinoma, Ref ECACC: 86010202), Huh-7D12 (differential hepatocellular carcinoma, Ref ECACC: 01042712), MDA-MB-231 (breast carcinoma, Ref ECACC: 92020424), HCT-116 (actively proliferating colorectal adenocarcinoma, Ref ECACC: 91091005), PC3 (prostate carcinoma, Ref ECACC: 90112714), NCI-H727 (lung carcinoma, Ref ECACC: 94060303) cell lines were obtained from the ECACC collection and HaCaT (keratinocyte from Cell Lines Service, Eppelheim, Germany). Cells were grown according to ECACC recommendations [23]. The toxicity test of the compounds on these cells was as follows: 2 × 103 cells for HCT-116 cells or 4 × 103 for the other cells were seeded in 96 multi well plates in triplicate and left for 24 h for attachment, spreading and growing. Then, cells were exposed for 48 h to increasing concentrations of the compounds, ranging from 0.1 to 25 mM in a final volume of 120 mL of culture medium. Cells were fixed in cooled ethanol-acetic acid solution (90:5 v/v), nuclei were stained with Hoechst 3342 (Sigma) and counted using automated imaging analysis (Cellomics Arrayscan VTI/HCS Reader, Thermo/Scientific). The IC50 were graphically determined.
Funding
One of us (A.B.) wishes to thank the “Ministère de l’Enseignement Supérieur et de la Recherche de Tunisie” for the grant. Financial support of this program carried out under the French cancer Institute “Cancéropôle Grand Ouest” in network “Marin molecules, Metabolism and Cancer” contract, is gratefully acknowledged.
Acknowledgments
The authors are grateful to the assistance of the staff (N. Le Yondre, P. Jéhan, F. Lambert) of CRMPO analytical chemistry core facility for HRMS analysis (CRMPO platform ScanMAT UMS 2001 CNRS, Université de Rennes 1, Bat. 11A, Campus de Beaulieu, Rennes, France).
Supplementary Materials
Supplementary materials can be found at: https://www.researchgate.net/profile/Jean_Bazureau.
Abbreviations
FTIR: Fourier Transformed Infra Red
HRMS: High Resolution Mass Spectrometry
NMR: Nuclear Magnetic Resonance
UV: Ultra Violet