Advances in Materials Physics and Chemistry
Vol.05 No.08(2015), Article ID:58585,6 pages
10.4236/ampc.2015.58029
Studies on AC Electrical Conductivity and Dielectric Properties of PVA/NH4NO3 Solid Polymer Electrolyte Films
Alabur Manjunath*, Tegginakeri Deepa, Naraganahalli Karibasappa Supreetha, Mohammed Irfan
Department of P.G. Studies in Physics, Government Science College, Chitradurga, India
Email: *manjugsc@yahoo.com
Copyright © 2015 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/



Received 24 May 2015; accepted 2 August 2015; published 5 August 2015
ABSTRACT
Solid polymer electrolytes have been extensively studied due to wide applications in various electrochemical devices [1] -[3] . Most of the solid polymer electrolytes consist of polymer as a host material to provide strength and good mechanical stability and an inorganic salt that supplies ionic carriers to cause electrical conductivity. In our studies, we prepared PVA/NH4NO3 polymer electrolyte films by solution casting method. The prepared films with varied Ammonium Nitrate concentration from 0 - 20 wt% are characterized by XRD & FTIR spectroscopy. XRD results show that amorphous nature increases as the amount of the Ammonium Nitrate salt in PVA is increased. IR-spectra confirm the polymer salt complexes in the range of 3700 - 712 cm−1. Conductance analysis reveals that polymer electrolyte films containing 20 wt% of NH4NO3 exhibit the highest ionic conductivity of 1.01 × 10−7 S/cm while pure PVA films give the lowest ionic conductivity of 2.10 × 10−11 S/cm. It was evident from this study that the increase of ionic conductivity depended on the Ammonium Nitrate salt concentration. The dielectric constant exponentially decreases with increase of frequency for pure PVA and NH4NO3 doped PVA film composites. The temperature dependent studies of AC conductivity and dielectric constant also included to understand the conducting property. The results obtained by these studies are reported in this work.
Keywords:
Polymer Electrolytes, XRD, FTIR, Electrical Conductivity

1. Introduction
Polymers form a very important class of materials without which life seems very difficult. Polymeric materials also have better mechanical properties for the construction of all practical solid-state electrochemical cells. Generally, the addition of inorganic salts into a polymer matrix can improve its conductivity. PVA is one of the most important polymer materials and it has many applications in industry, it exhibits good film forming property and enhances the conductivity. NH4NO3 is a white crystalline solid at room temperature and pressure. Commonly, used in agriculture as fertilizer. The present study is focused on the preparation and characterization of PVA-NH4NO3 polymer electrolyte films [4] -[6] .
2. Experimental Part
2.1. Materials and Preparation of Polymer Electrolyte Films
The chemicals used for the preparation are AR grade Polyvinyl alcohol (PVA) and Ammonium Nitrate (NH4NO3). Different compositions of Polyvinyl alcohol (PVA) and Ammonium Nitrate (NH4NO3) films have been prepared by solution casting method , using different weight ratios of NH4NO3 (0, 5, 10, 15, 20) wt%. The solution of PVA andNH4NO3 is obtained by dissolving them in distilled water at 350 K, and the solution is stirred well using magnetic stirrer for about one hour, until highly homogenous polymer solution was formed. These homogenous solutions were casted in a glass dish (diameter of 5 cm). The whole assembly was placed in a dust free chamber and the solvent was allowed to evaporate slowly in open air at room temperature for a week and films are peeled off from the glass dish. The thicknesses of the films were in the range of (0.03 - 0.18) mm [7] .
2.2. Instrumentation
In order to investigate the structure of polymer electrolytes, XRD studies of the films were carried out with an instrument RigakuMiniflex II X-ray diffractometer with CuKα radiation of λ = 1.5406 Å in the range of 2θ = 5˚to 30˚. The FTIR spectrum has been recorded by using IRspectraphotometer in the range of 500 - 4000 cm−1 at a resolution of 4 cm−1.
3. Results and Discussion
3.1. XRD Analysis
Figure 1 shows the XRD pattern of NH4NO3 doped polymer electrolyte. A broad peak around 19.29˚ is observed for pure PVA and has been found to be shifted in the complex systems. It is also observed for different concentrations of NH4NO3 added polymer films. The increase in the broadness of the peak reveals the amorphous nature of the complexed system. Peaks corresponding to NH4NO3 have been found to be absent in the complexes indicating the complete disassociation of salt in polymer matrix. Thus XRD analysis reveals the complex formation between the polymer and the salt [8] -[12] .
Figure 1. XRD Spectra of pure PVA and PVA/NH4NO3 polymer electrolyte films.
3.2. FTIR Studies
FTIR absorption spectra of pure PVA film and with different concentrations of NH4NO3 are shown in Figure 2.
The broad band observed between 3287.48 cm−1 - 3216.57 cm−1 are refers to the intermolecular hydrogen bonding and O-H stretching vibration (region I). The vibrational band observed between 2939.56 cm−1 - 3054.05 cm−1 is associated with C-H stretching from alkyl groups (region II), and the absorption peaks observed between 1731.07 cm−1 - 1753.91 cm−1 (region III) are due to the stretching C=O and C-O from acetate group.
The C-H stretching band of pure PVA has been shifted to higher wave number in doped polymer electrolytes [13] .
Figure 2. FTIR Spectra of pure PVA and PVA/NH4NO3 polymer electrolyte films.
Following Table 1 shows the peak assignments of pure PVA and various concentrations of NH4NO3 polymer films.
Table 1. Values of peaks, peak assignments of pure PVA and various concentrations of NH4NO3 polymer films.
3.3. AC Conductivity Studies
Complex impedance spectroscopy gives information on electrical properties of materials and their interface with electronically conducting electrodes. The Solid Polymer Electrolyte (SPE) films were cut into small discs (2 mm diameter) and sandwiched between two stainless steel electrodes under spring pressure. The AC conductivity of these films was measured in the frequency range from 50 Hz to 5 MHz using the HIOKI 3532-50 LCR Hi-tester which was interfaced to a computer. Measurements were also made at temperatures ranging from 305K to 373K. The measured conductance G(ω) from 50 Hz to 5 MHz was used to calculate the AC conductivity of the sample using the relation,
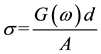
where, G(ω) is the measured conductance, A is the area of the sample and d is the thickness of the sample.
The variation of AC conductivity as a function of frequency for PVA composition of (PVA + NH4NO3) polymer electrolyte is shown in Figure 3. From Figure 3, it is observed that conductivity increases with increase in frequency. The increase in AC conductivity is due to increase in the composition of the salt in polymer matrix resulting in relatively more number of free ions. This will increase the mobile charge carriers as observed in Table 2. These charge carriers move in the amorphous polymer matrix and hence the conductivity increases. Thus there is a relation between the amorphous nature of the polymer film and the conductivity. In general, conductivity increases as the degree of crystallinity decreases, as observed above, which is the compliment of increase in amorphous nature [14] .
Figure 3. Variation of conductivity with frequency of pure PVA and NH4NO3 doped PVA polymer electrolyte films.
Table 2. Conductivity of pure PVA and NH4NO3doped PVA polymer electrolyte films.
3.4. Dielectric Studies
Figure 4 shows the variation of dielectric constant (ԑl) with frequency for different PVA concentration at room temperature. The permittivity (ԑl) was calculated using the relation,
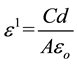
where, d is the thickness of the polymer film, A is the area of the electrolyte film, C is the capacitance of the cell with sample and
 = 8.85 × 10−12 F/m is the permittivity of free space.
= 8.85 × 10−12 F/m is the permittivity of free space.
Figure 4. Variation of Dielectric constant with frequency of pure PVA and NH4NO3 doped PVA polymer electrolyte films.
It is observed from the Figure 4 that ԑl(ω) decreases with increase infrequency and attain a constant value at higher frequency range similar to that for polar materials [15] [16] . The initial value of permittivity is high, butwith increasing the frequency, this value begins to decrease, which could be due to the dipole not being able to follow the field variation at higher frequencies [17] and due to polarization effects [18] . Further in the case of crystalline and semi-crystalline polymeric materials, the crystalline phase dissolves progressively into amorphous phase with increase in temperature. This in turn influences the polymer dynamics and thus the dielectric behavior. The low frequency region appears due to the contribution of charge accumulation at the electrode-elec- trolyte interface. At higher frequencies, the periodic reversal of the electric field occurs so fast that there is no excess ion diffusion in the direction of the field. It has been observed that dielectric permittivity decreases with increasing frequency in all the samples of PVA based polymer electrolyte films.
4. Conclusions
1) PVA based polymer electrolyte films with different concentrations of Ammonium Nitrate has been prepared by solution casting technique. The obtained film composites are solid, glassy to touch, transparent materials in appearance as shown in Figure 5.
2) The temperature dependence AC conductivity implies that for pure PVA and NH4NO3 doped PVA film composites, the AC conductivity increases with increase in frequency. It may be due to the increase of the mobility of charge carriers in the composite film. As the temperature increases from 40˚C to 70˚C in steps of 10˚C, it is observed that Dielectric constant exponentially decreases with increase of frequency as shown in Figure 6.
3) In XRD, a broad peak around 19.29˚ is observed for pure PVA and has been found to be shifted in the complex systems. It is also observed that for different concentrations of NH4NO3 added polymer films, the intensity of the peak decreases, and the full width at half maximum increases. This increase in the broadness of the peak reveals the amorphous nature of the complexed system. Peaks corresponding to NH4NO3 have been found to be absent in the complexes indicating the complete disassociation of salt in polymer matrix. Thus XRD analysis reveals the complex formation between the polymer and the salt.
FTIR Studies of pure PVA and NH4NO3 doped PVA exhibit formation of several bonds. The O-H (hydroxyl) stretching bond is the most characteristic stretching indicates the presence of hydroxyl group IR bond of alcohols. The free vibrations occur as a sharp peak at 3287.48 cm−1 in pure PVA, this peak is broadened in the doped PVA at 3264.70 cm−1, which is due to hydrogen bond formation. The wave number at 2939.56 cm−1 indicates an asymmetric stretching of C-H group which is shifted to 2942.22 cm−1 for NH4NO3 doped PVA film compo-
Figure 5. PVA + 5% NH4NO3.
Figure 6. Temperature dependent Dielectric constant with frequency for PVA/ 5% NH4NO3 doped polymer electrolyte films.
site. An absorption peak in pure PVA at 1731.07 cm−1 attributes to the C=O stretching mode and remains unaltered for the doped samples also. Similarly the absorption peak observed in pure PVA at 1660.06 cm−1 has been assigned to C-O in stretching mode in which it is shifted to 1633.97 cm−1 in doped samples. The FTIR studies indicate that NH4NO3 doped polymer film composites forms ionic bonds, molecular bonds weak anti bonding which may be indicated that unstable and stable complex structure.
Cite this paper
AlaburManjunath,TegginakeriDeepa,Naraganahalli KaribasappaSupreetha,MohammedIrfan, (2015) Studies on AC Electrical Conductivity and Dielectric Properties of PVA/NH4NO3 Solid Polymer Electrolyte Films. Advances in Materials Physics and Chemistry,05,295-301. doi: 10.4236/ampc.2015.58029
References
- 1. Qiao, J., Fu, J., Lin, R., Ma, J. and Liu, J. (2010) Alkaline Solid Polymer Electrolyte Membranes Based on Structurally Modified PVA/PVP with Improved Alkali Stability. Polymer, 51, 4850-4859.
http://dx.doi.org/10.1016/j.polymer.2010.08.018 - 2. Hema, M., Selvasekerapandian, S., Hirankumar, G., Sakunthala, A., Arunkumar, D. and Nithya, H. (2009) Structural and Thermal Studies of PVA: NH4I. Journal of Physics and Chemistry of Solids, 70, 1098-1103.
http://dx.doi.org/10.1016/j.jpcs.2009.06.005 - 3. Benedict, T.J., Banumathi, S., Veluchamy, A., Gangadharan, R., Ahamad, A.Z. and Rajendran, S. (1998) Characterization of Plasticized Solid Polymer Electrolyte by XRD and AC Impedance Methods. Journal of Power Sources, 75, 171-174.
http://dx.doi.org/10.1016/S0378-7753(98)00063-9 - 4. Leones, R., Sentanin, F., Rodrigues, L.C., Marrucho, I.M., Esperanca, J.M.S.S., Pawlicka, A. and Silva, M.M. (2012) Investigation of Polymer Electrolytes Based on Agar and Ionic Liquids. Polymer Letters, 6, 1007-1016.
http://dx.doi.org/10.3144/expresspolymlett.2012.106 - 5. Selvasekarapandian, S., Hema, M., Kawamura, J., Kamishima, O. and Baskaran, R. (2010) Characterization of PVA— NH4NO3 Polymer Electrolyte and Its Application in Rechargeable Proton Battery. Journal of the Physical Society of Japan, 79,163-168.
http://dx.doi.org/10.1143/JPSJS.79SA.163 - 6. Kadir, M.F.Z., Aspanut, Z., Majid, S.R. and Arof, A.K. (2010) FTIR Studies of Plasticized Poly(Vinyl Alcohol)-Chitosanblend Doped with NH4NO3 Polymer Electrolyte Membrane. Spectrochimica Acta Part A, 78, 1068-1074.
http://dx.doi:10.1016/j.saa.2010.12.051 - 7. Abdullah, O.Gh., Aziz, B.K. and Hussen, S.A. (2013) Optical Characterization of Polyvinyl Alcohol—Ammonium Nitrate Polymer Electrolytes Films. Chemistry and Materials Research, 3.
http://www.iiste.org/Journals/index.php/CMR/article/view/7045 - 8. Stephen, A.M., Saito, Y., Muniyandi, N., Ranganathan, N.G., Kalyanasundaram, S. and Elizabeth, R.N. (2002) Solid State Ionics, 148, 467.
http://dx.doi.org/10.1016/S0167-2738(02)00089-9 - 9. Fernandez, A., Torrecilla, J.S., Garcia, J. and Rodriguez, F. (2007) Thermophysical Properties of 1-Ethyl-3-methyli- midazolium Ethylsulfate and 1-Butyl-3-methylimidazolium Methylsulfate Ionic Liquids. Journal of Chemical & Engineering Data, 52, 1979-1983.
http://pubs.acs.org/doi/abs/10.1021/je7002786
http://dx.doi.org/10.1021/je7002786 - 10. Singh, M.P., Singh, R.K. and Chandra, S. (2010) Effect of Ultrasonic Irradiation on Preparation and Properties of Ionogels. Journal of Physics D: Applied Physics, 43, 4.
- 11. Bhargav, P.B., Mohan, V.M., Sharma, A.K. and Rao, V.V.R.N. (2009) Investigations on Electrical Properties of (PVA:NaF) Polymer Electrolytes for Electrochemical Cell Applications. Current Applied Physics, 9, 165-171.
http://dx.doi.org/10.1016/j.cap.2008.01.006 - 12. Kurumova, M., Lopez, D., Benavente, R., Mijangos, C. and Pevena, J.M. (2000) Effect of Crosslinking on the Mechanical and Thermal Properties of Poly(Vinyl Alcohol). Polymer, 41, 9265-9272.
http://dx.doi.org/10.1016/S0032-3861(00)00287-1 - 13. El-Hefian, E.A., Nasef, M.M. and Yahaya, A.H. (2010) The Preparation and Characterization of Chitosan Poly(vinyl alcohol) Blended Films. E-Journal of Chemistry, 7, 1212-1219.
http://dx.doi.org/10.1155/2010/626235 - 14. Hou, W.H., Chen, C.Y. and Wang, C.C. (2004) Conductivity, DSC, and Solid-State NMR Studies of Comb-Like Polymer Electrolyte with a Chelating Functional Group. Solid State Ionics, 166, 397-405.
http://www.sciencedirect.com/science/article/pii/S0167273803005307
http://dx.doi.org/10.1016/j.ssi.2003.09.021 - 15. Ramesh, S. and Chai, M.F. (2007) Conductivity, Dielectric Behavior and FTIR Studies of High Molecular Weight Poly(vinylchloride)—Lithium Triflate Polymer Electrolytes. Materials Science and Engineering: B, 139, 240-245.
http://dx.doi.org/10.1016/j.mseb.2007.03.003 - 16. Ramya, C.S., Savitha, T., Selvasekharapandian, S. and Hiran Kumar, G. (2005) Transport Mechanism of Cu-Ion Conducting PVA Based Solid-Polymer Electrolyte. Ionics, 11, 436-441.
http://dx.doi.org/10.1007/BF02430262 - 17. Ramesh, S., Yahana, A.H. and Arof, A.K. (2002) Dielectric Behaviour of PVC-Based Polymer Electrolytes. Solid State Ionics, 152-153, 291-294.
http://dx.doi.org/10.1016/S0167-2738(02)00311-9 - 18. Tareev, B. (1979) Physics of Dielectric Materials. MIR Publications, Moscow.
NOTES
*Corresponding author.









