Open Journal of Applied Sciences
Vol. 3 No. 2 (2013) , Article ID: 33392 , 15 pages DOI:10.4236/ojapps.2013.32031
The Limnology of Ohana Lake, a Potential Manmade Aquaculture System in Nigeria
Institute of Oceanography, University of Calabar, Calabar, Nigeria
Email: ajapaulo@yahoo.com
Copyright © 2013 Paul O. Ajah. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received February 15, 2013; revised March 18, 2013; accepted March 26, 2013
Keywords: Limnology; Aquaculture; Productivity; Pollution; Fishery; Benthos
ABSTRACT
The concentrations of heavy metals (Fe > Zn > Cu > Pb > Ag) in bottom sediments and fish gills in Ohana Lake, were found to be significantly high and far exceeded FEPA and WHO environmental standards for water quality by 1.5 to 18 times, respectively. Six classes of each of phytoplankton and zooplankton with a total of 35 phytoplankton taxa comprising 46 species i.e. 35(46) and 22(28) faunal were observed. The class Chlorophyceae dominated the phytoplankton community with 18(22) followed by Cyanobacteria 6(10). The aquatic fauna was dominated by the Rotifera 8(11), followed by the Copepoda 6(9). The benthic flora community consisted of five classes of phytoplankton made up of 28(36). The class Bacillariophyceae 11(15) dominated the group followed by Chlorophyceae 10(11). Benthic fauna were made up of seven classes of 13(13). The dominant class Nemata 4(4) was followed closely by Protozoa 2(3). Ohana Lake is fast turning to a eutrophic ecosystem with accompanied algal bloom due to very high nutrient contents. The equitability or evenness indices (J) for both phytoplankton and zooplankton were lowly indicating generally low species diversities as well as predominantly unstable ecosystem. The aquacultural implications of these parameters are discussed.
1. Introduction
Residential development of lakeshores is expected to change a variety of key lake features that included increased nutrient loading, increased invasion rate of nonnative species, increased exploitation rates of fishes by anglers, and alteration of littoral habitats. All of these factors may alter the capacity of lakes to support productive native fish populations [1]. Ohana Lake being quite close to residential area witnesses a lot of anthropogenic activities such as bathing; canoeing and car/motorcycle wash, and consequently, is prone to suffer such fate.
Heavy metals are considered as major environmental pollutants and are regarded as being cytotoxic, mutagenic and carcinogenic [2].
Knowledge of the physicochemical regimes of a body of water is invaluable in the determination of the productivity and other characteristics [3,4] opined that fertility of water is related to its chemical properties and an understanding of the water chemistry serves as the basis for determining whether the water is rich or poor in biological production. Physicochemical factors are known to influence vertical and horizontal migration of organismsthe distribution and feeding regime [5]. The water quality of the lakes and rivers is assessed by the physicochemical and biological characteristics of their waters. It is known that several water quality parameters if not within normal range could act as stressors and adversely affect fish growth and reproduction [6]. Hence, regular monitoring of physicochemical factors is essential for assessing the status of lakes with references to fish culture [7,8] noted that density and diversity of flora and fauna were dependent on chemical regime of water. Fish yields from lakes and reservoirs are known to have strong correlation with primary production [9,10]. [11] studied the concentration of heavy metals in water and Hemichromis fasciatus of a waste pit influenced by petroleum activities and found that, seasonally, Cd, Cu, Pb, Mn, Co, Cr, V and Hg had higher mean values in water during the dry season while Zn, Ni and Fe were higher during the raining season. All heavy metals except Fe were higher in mean values during the dry season in H. fasciatus than during the rainy season.
A survey of the benthos makes it possible to establish if pollution has occurred in recent past and, if so, whether toxic or organic in nature. When organic pollution has occurred, the number of species is usually restricted, although the few species present may be present in very high numbers. On the other hand, pollution from toxic substances may eliminate almost all animals present except for a few highly resistant species. A survey of the benthos is, therefore often of more use than an analysis of a water sample, since the water sample represents only one sample taken at one particular point in time and tells you little about the integrated, long-term effects of water quality [12].
The study of Ohana Lake is aimed at assessing its limnology and possible aquacultural potentials. Studies like this make significant contribution to fishery management by identifying potential damage to fish populations, through low oxygen levels, toxic blooms or anthropogenic pollution [13].
Description of the Study Area
Ohana Lake is located between latitudes 05˚57''24''N and 05˚57''31''N and longitudes 008˚22'38''E and 008˚22'46''E. It is situated barely 30 m away from the major road linking Calabar and Ikom town and right at the centre of Ohana community in Obrubra Local Government area of Cross River State, Nigeria (see Figure 1).
Ohana Lake could be characterized as an oligotrophic perennial lake due to its nutrient poor status. It is also a limnetic lake due to its lack of rooted vegetation. The Lake covers an area of 3.0 km2. The average depth of the Lake was 4.72 m with maximum depth of 6.5 m and minimum 0.3 m with a water volume of 14.173377 km3. It is of low lying vegetation comprising of shrubs and a few permanent trees surrounding it. Lemna minor dominated aquatic macrophyte.
2. Materials and Methods
Ohana Lake was sampled fortnightly in 2005 in the month of March. Sampling locations were gotten using a GPS instrument and the reading converted from longitudes and latitudes to Universal Traverse Mercado (UTM) coordinate system. Various water depth positions were first measured using a calibrated line with a lead and latter confirmed using an echo sounder. Heavy metals were analyzed using atomic absorption spectroscopy (A.A.S) following Unicam 919 Solar System. The fish species were identified using [14] while the mollusks were identified using [15]. Both phytoplankton and zooplankton were identified using [16-18]. Primary production of the lake was determined using light and dark BOD bottles at various depths and days in accordance to [19]. The benthic communities were first fractionated using screens with the following ranges; 0.105 mm, 0.50 mm, 0.30 mm, 0.50 mm and 0.80 mm stacked together and shaken vigorously and severally under a slow flowing tap water and latter identified under a microscope for the smaller groups and direct observation for the macro benthos using the appropriate taxonomic keys. Dissolved oxygen and temperature were analyzed by meter/probe methods [20], nitrate by cadmium reduction/diazotization method following [21]. Phosphate was analyzed by molybdenum blue method (spectrophotometrically) [21]. Total dissolved solids (TDS) and conductivity by meter/ probe methods (3000 HACH); turbidity was by transparency disc while Total suspended solids (TSS) and colour was measured using spectrophotometer (3000 HACH). Water hardness—Ca2+, Mg2+—by complexometric (titration) method [20,22].
The Shannon-Wiener’s index (H1) was adopted in calculating the diversity indices of species (ShannonWiever, 1948) while the Equitability or evenness index (J) was used to compare the H1 index.
3. Results
Iron (Fe) values from sediment sample averaged 359.8 mg/L. Likewise, the average values 10.01 mg/L, 10.79 mg/L, 5.9 mg/L and 37.42 ml/L, respectively, were realized for lead, copper, silver and zinc in the sediments. Results of analysis of fish gills showed that lead was not detected, the rest metals had the following values: iron— 35.17 mg/L, copper—0.315 mg/L, silver—0.45 mg/L and zinc—3.097 mg/L. The summary of the trace metals of Ohana Lake are represented in Table 1.
Table 2 summarizes the physicochemical parameters and nutrients of Ohana Lake, Nigeria during the sampling periods. The hydrographic data and nutrients in Ohana Lake are shown in Table 3. Temperature ranged from 31.7˚C to 35.0˚C with average of 33.12˚C ± 1.01˚C. Ohana Lake exhibited a mild thermal stratification. Both temperature and dissolved oxygen decreased with depth. Dissolved oxygen levels in Ohana Lake ranged from 3.9 to 4.1 mgO2/L with a mean of 4.01 ± 0.099 mgO2/L. Figure 2 shows the inverse relationships between dissolved oxygen and depth in metres in Ohana Lake. The pH of the water ranged from 6.90 to 7.79 with average of

Table 1. The mean values of the trace metals of Ohana Lake, Cross River State, Nigeria on fish and sediment samples.


Figure 1. Map of Cross River State, Nigeria, showing the sampling location—Ohana Lake located at Lat. 05˚57'24''N and 05˚57'31''N and long. 008˚22'38''E and 008˚22'46''E—by an arrow.
7.66 ± 0.28 (Table 3). The total alkalinity in Ohana Lake ranged from 20 to 25 mg/L with a mean of 21 ± 2.1 mg/L. The values for total hardness ranged from 16.0 to 25.9 mg/l with a mean of 19.61 ± 2.1, while that of conductivity was 33.0 ± 6.46 μs/cm ranging from 29.0 to 48.0 μs/cm. TDS ranged from 14 to 26 with a mean of 16.2 ± 3.64, nitrate was from 0.231 to 1.98 mg/l with a mean of 0.645 ± 0.54, sulfate ranged 1.170 to 1.81 mg/l and averaged 1.517 ± 0.17, silicate ranged from 1.149 to 1.386 mg/l and had a mean of 1.208 ± 0.10 while colour ranged from 28 to 45 pt/Co with a mean of 33.2 ± 9.58.
Plankton Distribution, Density and Diversity
The water body had six classes each of phytoplankton

Table 3. Mean hydrographic data of Ohana Lake, Cross River State, Nigeria.

Figure 2. Relationship between depth and dissolved oxygen in Ohana Lake in Cross River State, Nigeria.
and zooplankton with a total of thirty-five phytoplankton taxa comprising forty-six species and twenty-two faunal taxa of twenty-eight species. The class Chlorophyceae dominated the phytoplankton community with eighteen taxa having twenty-two species followed by Cyanobacteria had six taxa comprising ten species and Bacillariophyceae with six taxa and seven species. Both Pyrrophyceae and Xeminophyceae had three taxa and three species each while Euglenophyceae had one species from one taxon. The zooplankton was dominated by Rotifera of eleven species from eight taxa, followed by the Copepoda with six taxa comprising nine species. Both Cladocera and Ostracoda came third in abundance having three taxa and three species each, and lastly Protozoa with two taxon and two species (Table 4).
The benthic flora had five classes of phytoplankton made up of twenty-eight (28) taxa and thirty-six (36) species. The class Bacillariophyceae dominated the group with eleven (11) taxa of fifteen (15) species followed by Chlorophyceae with eleven species from 10 taxa. Cyanobacteria followed this with six (6) taxa and eight (8) species (Table 4). The other two classes Euglenophyceae and Xeminophyceae each had one taxon and one species. The aquatic benthic fauna represented by seven classes of 13 taxa and 13 species was dominated by the class Nemata of 4(4) followed by Protozoa 2(3), Rotifera 2(2), while Bivalvia (Protobranchia); Cladocera, Gasterotricha, Odonata and Ostracoda had one taxon and one species each.
The mean diversity index for the water flora of Ohana Lake was 0.969 ± 2.55 and 0.778 ± 1.23 for zooplankton. The equitability or evenness index (J) yielded 1.611 and −3.05, respectively for phytoplankton and zooplankton (Table 3). These low diversity indices indicate generally low species diversities as well as predominantly unstable ecosystem. The diversity index for the benthic flora was 0.371 ± 0.57 while that of the benthic fauna was 2.23 ± 2.80. The equitability or evenness index (J) resulted in −15.50 and 4.18, respectively, for benthic flora and fauna. The very low floral diversity index values indicate very low species diversities as well as predominantly unstable ecosystem while the faunal diversity indexes indicate slightly higher species diversity and a mild stable ecosystem.
The planktonic community present in the water column of Ohana Lake included the following: Bacillariophyceae (Coscinodiscus excentricus, Ehr, C. lacustris Grunow, Cyclotella stelligera l et Grun, Fragilaria sp. Melosira ambigua (Grun), Surirella robusta var splendid, Thermalis denticum), Chlorophyceae (Chlorella vulgaris Beij, Chlamydomonas sp, Chodatella wratislawiensis (Shroeder), Coeolosphaerium dubium, Cosmocladium saxonicum De Bary, Crucigenia rectangularia (Näg.), Eudorina elegans Ehr., Lauterboniella elegantissima Schmidle, Lyngbya limnetica Lemm., Pediastrium sturmii Reinsch, Protococcus sp, Scenedesmus bijuga (Turp.), S. obliquus (Turp.), S. quadricauda Bréb, Spirogyra sp, Staurastrum apiculatum Bréb, Tetradesmus wisconsinensis Smith, Tetrastrum elegans Playfair, T. heterocanthum (Nordst.), Tetraedron tumidulum Hansg, T. minimum (A.Br.), Zygnema sp, Cyanobacteria (Anabaena affinis Lemm, Aphanothece clathrata W. et G.S. West, Aphanothece stagnima B. Peters & Geitl, Coelosphaerium kuetzingianum Näg, Gloeocapsa limnetica (Lemm.), Merismopedia punctata Meyen, Microcystis aeruginosa Kurz, Microcystis pulverea (Wood), Microcystis aeruginosa F. Flos-aquae, Microcystis Grevillei Hass,), Euglenophyceae Euglena caudate Hübner), Pyrrophyceae (Glenodinium cinctum Ehr, Gymnodinium excavatum Nyg, Peridinium cinctum (O.F.M), Xeminophyceae (Tribonema minus Hazen, Tribonema viride Pasch,




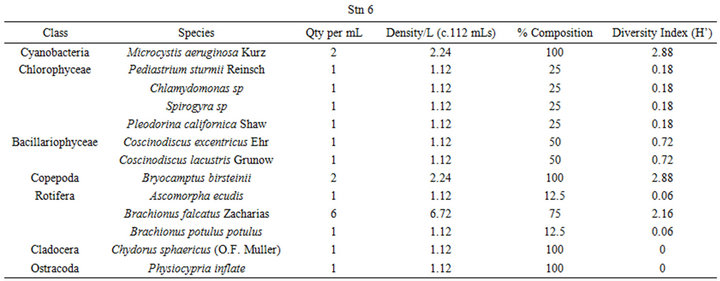

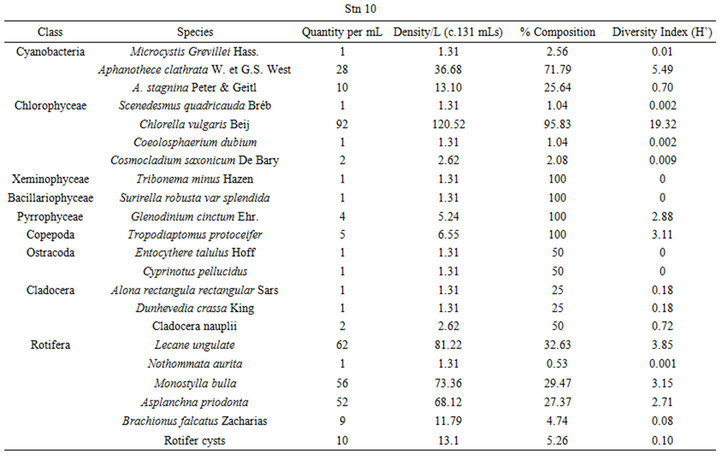

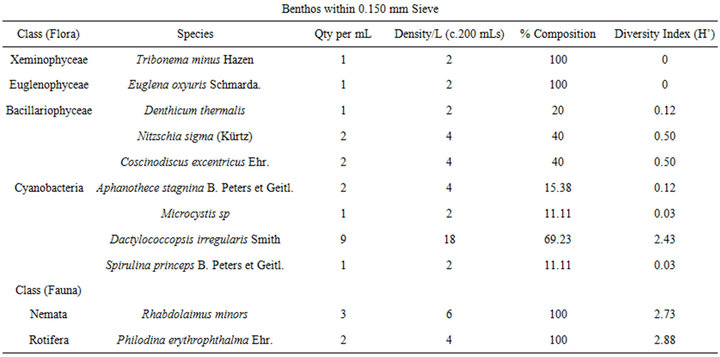




Table 4. Shows plankton and benthos distribution, density, percentage composition and diversity indices at the given sampling stations in Ohana Lake.
Tribonema vulgare Pasch) while the Zooplankton community included: Copepoda (Bryocamptus birsteinii, Eudiaptomus gracilis, Heterocape appendiculata Sars, Microcyclops rubellus, Microcyclops varicans Sars, Thermocyclops crassus, Thermocyclops kawamurai Kikuchi, Thermocyclops neglectus Sars, Tropodiaptomus protoceifer, Cop. Nauplii), Protozoa (Arcella vulgaris Ehr., Stentor polymorphus Müller), Rotifera (Ascomorpha ecudis, Asplanchna priodonta, Brachionus calyciflorus dorcas (Goose), Brachionus falcatus Zacharias, Brachionus potulus potulus, Lecane luna Müller, Lecane ungulate, Lepadella patella Müller, Monostylla bulla, Nothommata aurita, Rotifer cysts, Testudinella caeca,) Cladocera (Chydorus sphaericus (O.F. Muller), Graptoleberis testudinaria Fischer, Dunhevedia serrata Daday, and Ostracoda (Cyprinotus pellucidus, Entocythere talulus Hoff, Physiocypria inflate)
Similarly the benthic floral and faunal of Ohana Lake consisted of Bacillariophyceae (Achnanthes inflate, A. lanceolta, A. Peragallii, Coscinodiscus excentricus Ehr, Cymbella naviculiformis Auerswald, Denthicum thermalis, Gomphonema sphaerophorum, Gyrosigma acuminatum (Kürtz.), Nitzschia sigma (Kürtz), Pinnularia Braunii, P. appendiculata (Ag.), Stauroneis anceps Ehr, Surirella Capronii Bréb, Surirella robusta Ehr., Surirella robusta var. splendida), Chlorophyceae (Closteriopsis longissima var. tropica W. & G. west, Closterium strigosum Bréb, Eudorina elegans Ehr., Hyalotheca dissiliens (Sm.), Lyngbya limnetica Lemm., Pediastrum boryanum Menegh., Scenedesmus armatus (Chod.), S. bijuga (Turp.), Spirogyra sp Tetradesmus wisconsinensis Smith. Tetrastrum heterocanthum (Nordst.), Cyanobacteria (Anabaena sp., Aphanothece clathrata W. et G.S. West, A. stagnina B. Peters et Geitl., Dactylococcopsis irregularis Smith, Microcystis Grevillei, Microcystis sp., Oscillatoria limosa Ag., Spirulina princeps B. Peters et Geitl.,) Euglenophyceae (Euglena oxyuris Schmarda.), Xeminophyceae (Tribonema minus Hazen)
The Benthic fauna species diversity in Ohana Lake Included Cladocera (Chydorus sphaericus (O.F.Muller), Bivalvia (Nucula sulcata Bonn), Gasterotricha (Chaetonotus sp), Nemata (Aphelenchoide microlaimus, Diplogaster factor, Rhabdolaimus minors, Trilobus longus), Ostracoda (Physiocypria inflate), Odonata (Neurocordulis sp.), Protozoa (Chilodonella spp Chilodonella uncinata, Paramecium caudatum Ehr,), and Rotifera (Eothinia elongate Ehr. Philodina erythrophthalma Ehr.).
The net photosynthetic activity ranged from −0.0312 C in mg/L to 0.312 C in mg/L with a mean of −0.063 ± 0.277 C in mg/L while the gross photosynthetic activity ranged from 0.0 joules (j) to 23.13j with a mean of 7.44 ± 10.50j. Net primary production at surface (0.0 m), middle (0.5m) and 1.0m depth, respectively, for four samplings were −0.0312 C in mg/L, −0.412 C in mg/L, −0.686 C in mg/L; −0.312 C in mg/L, 0.094 C in mg/L, 0.094 C in mg/L; 0.062 C in mg/L, 0.062 C in mg/L, −0.125 C in mg/L; and 0.312 C in mg/L, 0.094 C in mg/L, 0.094 C in mg/L. The corresponding Gross photosynthetic activity were 10.28 energy in joules (j), 3.855j, 0.0j; 2.57j, 23.13j, 10.28j; 2.57j, 2.57j, −14.778j; and 15.42j, 23.13j, 10.28j. Positive correlation (r = 0.504) was observed between net and gross photosynthetic activity in Ohana Lake. Decreasing photosynthetic activity was observed when the light and dark bottles were allowed to stay beyond the period of active photosynthesis (10 a.m. to 4.00 p.m.).
4. Discussion
The concentration of heavy metals in the fish gills and bottom sediments from Ohana Lake were found to be significantly high and exceeded the water quality criteria by 11/2 to 18 times, respectively. Besides lead that was not detected in fish, iron, copper, silver and zinc all exceeded the [23] set standards in both fish and sediments. Iron values were beyond the 20 mg/L upper limit for polluted environment [23-25]), which puts Ohana Lake as a highly polluted environment. Comparatively, the concentration of heavy metals in the interstitial water was found to be significantly high and exceeded the water quality criteria by three to eleven times [26]. [11] opined that the mean abundance of heavy metals in H. fasciatus was Fe > Zn > Mn > Cu > Cd > Co > Pb > Ni > Cr > V. The monthly concentrations of heavy metals in H. fasciatus were far higher than those in water. The concentrations of Pb, Fe and Mn were above WHO limits for drinking water for some months while Fe and Mn values in H. fasciatus were above WHO limits for food in twelve months with Zn, Cd, Cu, Pb, Ni, Co and Cr exceeding WHO limits for some months. The findings reveal that the water and the fish (H. fasciatus) are contaminated and not fit for drinking and consumption by humans. The heavy metal pollution of natural environment has been consistently increasing through effluents, sedimentation of rocks and mining activities [2]. The high load of heavy metals in Ohana Lake must have been due to accumulation of heavy metals from the explosives used during quarrying activities and sedimentation of rocks and wash off from cars etc. [27] indicated that the general pattern in descending order of heavy metals accumulation in the muscle of the fishes examined is Fe > Zn > Cu > Pb. The highest mean value of heavy metals recorded is the iron level in Scomber scombrus (944.38 + 548.58 mg/kg). The concentration of iron and copper were significantly different (P < 0.05) among the fish samples. The mean value of Iron in Scomber scombrus samples examined was higher than the World Health Organization (WHO) allowable limit in food.
The presence of metal pollutants in freshwater is known to disturb the balance of aquatic ecosystem and this has been noticed to manifest in the presence of some irregularities in fish physiology as fishes tend to concentrate some metals in their body tissues [28]. It was also recognized that the increased environmental burdens of metals and acids in lakes were potentially stressful to local fisheries. For instance, fish population may be lost from lake apparently because of reproductive failures [29]. Thus, fishes are sensitive indicators of heavy metals pollution [30]. Heavy metals such as lead, cadmium, zinc, iron, silver, mercury and chromium etc have not been shown to be essential for life. Rather they are introduced into our environment in large quantities by human activities. Organometalic derivatives of these metals are usually volatile and may be concentrated in the fatty tissues, and sometimes result in chromosomal damage thus making them particularly dangerous. [31] noted that the use of fish alone to access the level of heavy metals may be misleading if not carefully interpreted.
The temperature range of Ohana Lake fell within the recommended temperature conducive for optimum growth of fish in the tropics according to the Institute of Food and Agriculture Sciences, University of Florida (IFAS, Circular 1051). The study shows that the lake water temperature remained around the upper limit of the optimum range throughout the sampling periods and hence might not probably affect fish growth. However, [32] observed a milder water temperature in Lake Chad to be 24.63˚C ± 4.70˚C, which must have contributed to the high productive nature of Lake Chad compared to the low productive nature of Ohana Lake.
The dissolved oxygen level in Ohana Lake was lower than that of Lake Chad (6.95 ± 0.53 mgO2/L) [32]. Low DO level, indicates decomposition of organic matter and bacterial and may be caused by high temperature leading to decreased oxygen holding capacity of water as suggested by [33] for Senthil sarovar and due to higher trophogenic activities as observed in Lake Taudaha in India [34]. The minimum DO level required in a lake to support average or good fish production is >5 ppm [35]. Both the range and mean DO in Ohana Lake fell below the minimum, and consequently, is not likely to support good fish growth.
[35] Earlier stated that both highly acidic and alkaline medium (pH 5.50 to 6.50 and pH 9.0 and above) is unsuitable for good fish production. [36-38] indicated that alkaline medium is a usual attribute of a productive water bodies. [36] and [39] reported the normal range for inland waters as 6.0 to 9.0. The pH of Ohana Lake is within this range and hence not likely to constitute stressor for fish.
Generally, the minimum level of total alkalinity required for water to be most productive is >50 ppm [40]. The minimum level of total alkalinity preferred for fish culture is 100 mg/L [41]. The very low level of total alkalinity (20 to 25 mg/L) in the lake is unlikely to support aquaculture growth.
Total hardness fell within WHO/EEC standard. [42] stated that a lake is adjudged to be distinctly eutrophic when the calcium exceeds 25 mg/L. The only station (stn 5) with 25.9 + 0.001 beyond this range was a small channel outside of the main lake but has some connection with the lake such that during high water level there is a mixing of the two water bodies.
[32] had mean conductivity in Lake Chad as 380.63 ± 51.76. The very low electrolyte conductivity of the Lake which depicts low concentration of ions (i.e. charged solutes) compared to the range of 21.5 to 1523 μs/cm with a mean of 493.6 ± 432.5 μs/cm in outdoor condition by [43] infers that Ohana Lake is unlikely to promote good aquaculture activity.
Turbidity is caused by wide variety of suspended solids, organic colloidal compounds and cause dispersion of sewage [44,45]. Turbidity reduces the light penetration and high rate of phytoplankton production is restricted to the upper waters. With water transparency using a secchi disc ranging from 0.8 m to 1.4 m with a mean of 1.0 ± 0.28 m, the secchi depth was seen to be inversely related to turbidity and determined the conditions of availability of light in the water column to support photosynthesis by phytoplankton, and hence primary production. The approximate vertical extent of the euphotic zone in Ohana Lake is 3.0 m following [43,46] had a mean of 90.47 ± 65.2 FTU, which promoted good fish culture.
The higher the silicate levels the more the diatoms. The low values are reflected in the low diatom group in the sample area.
The value for total dissolved solids in Ohana Lake is far below the upper limit set by [23] for polluted environment. Suspended solids or particulate matter in fresh or marine waters are of importance to aquaculture because they may damage fish gills and interfere with respiration. Secondly, they may cause siltation and smothering of benthos and interference with feeding of bivalve filter feeders. High turbidity due to suspended solids also reduce photosynthesis and hence production of phytoplankton and submerged periphyton. A high content of suspended organic solids will exert a biological oxygen demand and lead to oxygen depletion. Based on [23] guidelines the total dissolved solids in Ohana Lake are much less than the upper limit set that could cause pollution.
BOD5 levels usually depict the organic enrichment and show the decay of plants and animal matter in the Lake [47]. Water bodies with BOD5 higher than 35 - 45 mg/L are not to be considered as good quality for fish culture [48]. The BOD5 of Ohana Lake is comparatively quite low, and consequently, is not likely to contribute adversely to fish growth and culture. The minimum level of nitrate required for a lake to be productive is 0.1 mg/L [7]. The mean value of 0.645 ± 0.54 mg/L in Ohana Lake is much higher than the minimum requirement and is likely to have contributed to the algal blooms observed in the lake.
The phosphate levels in the lake were zero in all the sampling stations. The apparent absence of phosphate may be due to the immediate utilization of nutrients by the phytoplankton. The absence of  may also be due to the interaction between
may also be due to the interaction between  and calcium [49]. [50] indicated that the optimum level of phosphate needed for the growth of plankton is 0.1 to 0.2 mg/L. When higher than this, may lead to eutrophication and algal blooms [51] and could be detrimental to fish. The present study has zero level phosphate concentration, which may be due to its utilization by plants to generate intense algal bloom. However these zero values are far below the optimum required for plankton growth required to form enough food for fish, and consequently, may not be able to boost the fish community of the Lake.
and calcium [49]. [50] indicated that the optimum level of phosphate needed for the growth of plankton is 0.1 to 0.2 mg/L. When higher than this, may lead to eutrophication and algal blooms [51] and could be detrimental to fish. The present study has zero level phosphate concentration, which may be due to its utilization by plants to generate intense algal bloom. However these zero values are far below the optimum required for plankton growth required to form enough food for fish, and consequently, may not be able to boost the fish community of the Lake.
Highly coloured water is not desirable on aesthetic ground and may not be suitable for some industrial uses [52]. Apparent colour in Ohana ranged from 16 pt/Co to 45 pt/Co with a mean of 35.3 ± 9.1 pt/Co. At all sampling station the apparent colour in Ohana Lake is beyond the [23] standard of 7. These high colour values put Ohana Lake as a polluted aquatic medium.
[53] observed a total of 13 species of green algae, 14 species of blue-green algae and 24 species of diatoms in three water supply reservoirs in Tamilnadu.
The mean diversity index for the water flora of Ohana Lake was 0.969 ± 2.55 and 0.778 ± 1.23 for zooplankton. The equitability or evenness index (J) yielded 1.61 and −3.05, respectively, for phytoplankton and zooplankton. These low diversity index values indicate generally low species diversities as well as predominantly unstable ecosystem.
Fish yields from lakes and reservoirs are known to have strong correlation with primary production [9,10]. In aquaculture systems algal production, as measured by primary production, is also a good indicator of fish yields, although the relationship to fish yield is complicated by input of feed and other organic matter, such as manure. These may be ingested directly by the fish or channeled along the detritus pathway, thus boosting benthic production and indirectly the production of benthos feeding fishes. These values are far below the optimal levels 0.742 to 1.645 mgO2/L (mean 0.771 ± 0.509 mgO2/L) with average energy in joules of 28.108j to 60.788j (mean 30.886 ± 20.05j) as determined in fishponds by [43] suggesting that Ohana Lake is indeed oligotrophic.
When the light and dark bottles were kept beyond the photosynthetically active radiation, the initial DO became higher than the final DO and resulted into negative net primary production. Net photosynthesis decreased with depth.
A dimension to the problem of europhication is excessive growth of planktonic and attached algae and other aquatic macrophytes (water weeds), which exerts significant deleterious effects on the beneficial use of lakes [53]. [53] observed the presence of Oscillatoria sp., Anabaena sp. and Gomphonema sphaerophorum as the dominant form and concluded that the system was eutrophic. Similarly, the presence of these species including Microcystis sp and Aphonothece sp. in all the sampling stations in large amounts, in addition to three species of Pyrrophyceae, Glenodinium cinctum, Peridinium cinctum (O.F.M) and Gymnodinium excavatum often indicators of polluted environment must have contributed to the eutrophication of Ohana Lake. Potential and realized water quality deterioration is associated with excessive growth of algae and other aquatic plants, and alters the physicochemical factors of water [53]. Excessive growth due to eutrophication is known to release extra cellular products capable of acting as trihalomethanes (THM) precursors [54]. Also, Daum (USEPA, 1979-unpublished report, as cited by [54] reported that Anabaena and Pandorina morum serve as THM precursors when exposed to chlorination. [53] found Anabaena sp. as the chief component of algal community and frequently recorded algal blooms. One other factor that must have contributed to the pollution of the lake must have been the presence of aquatic weed Lemna minor in large amount around the Lake’s small outlet. Finally, a look at the benthic community shows the dominance by the class Nemata. These nematode worms are often found in eutrophic bottoms.
The presence of diatoms Melosira sp. and Coscinodiscus excentricus are indications of polluted water bodies. Likewise Philodina sp (a Rotifera) is often associated with polluted waters.
The diversity index for the benthic flora was 0.371 ± 0.57 while that of the benthic fauna was 2.23 ± 2.80. The equitability or evenness index (J) resulted in −15.50 and 4.18, respectively, for benthic flora and fauna. The very low floral diversity index value indicates very low species diversities as well as predominantly unstable ecosystem while the faunal diversity index indicates slightly higher species diversity and a mild stable ecosystem.
The high load of heavy metals in Ohana Lake must have been due mainly to anthropogenic activities emanating from accumulation of heavy metals from the explosives used during quarrying activities, sedimentation of rocks and car wash wherein car paints, oil and grease and metal were introduced into the system.
5. Conclusions
Residential development of lakeshores is expected to change a variety of key lake features that included: increased nutrient loading, increased invasion rate of nonnative species, increased exploitation rates of fishes by anglers, and alteration of littoral habitats. All of these factors may alter the capacity of lakes to support productive native fish populations [1]. Ohana Lake being quite close to residential area witnesses a lot of anthropogenic activities such as bathing; canoeing and car/motorcycle wash and consequently are prone to suffer this fate.
Both trace metals from the sediment and fish gills proved Ohana Lake to be a highly polluted aquatic environment. Furthermore, Ohana Lake is fast turning to a eutrophic ecosystem with accompanied algal bloom due to very high nutrient contents (NO3 etc.). The planktonic community was dominated by algae, in particular, Microcystis spp, Oscillatoria sp., Aphanothece spp., Anabaena sp., Gomphonema sphaerophorum, Glenodinium cinctum, Peridinium cinctum (O.F.M) and Gymnodinium excavatum often known as indicators of aquatic pollution. The fish species observed during the sampling period were basically Tilapia galilea and Oreochromis niloticus and were of small sizes probably due to stress from pollution.
6. Acknowledgements
The author wishes to thank the Food and Agriculture Organisation (FAO) for providing the fund for carrying out this research.
REFERENCES
- D. E. Schindler, S. I. Geib and M. R. Williams. “Patterns of Fish Growth along a Residential Development Gradient in North Temperate Lake,” Ecosystems, Vol. 3, No. 3, 2000, pp. 229-237. doi:10.1007/s100210000022
- S. Punita and S. C. Gajaria. “Feasibility and Utility of Green Algae for Metal Remedy,” Indian Hydrobiology, Vol. 7, No. 1-2, 2004, pp. 85-88.
- A. A. Adebisi, “The Physicochemical Hydrology of a Tropical Seasonal River, Upper-Ogun River,” Hydrobiologia, Vol. 79, No. 2, 1981, pp. 157-165. doi:10.1007/BF00006123
- A. M. A. Imevbore, “The Chemistry of the River Niger in the Kainji Reservoir Area,” Arch. Hydrobiologia, Vol. 30, 1970, pp. 154-176.
- I. F. Adeniyi, “Studies of the Physicochemical Factors and the Planktonic Algae of Lake Kainji, Nigeria,” Ph.D. Thesis, University of Ife, Ile-Ife, 1978.
- G. K. Iwama, M. M. Vijayan and J. D. Morgan, “The Stress Response in Fish,” Ichthyology, Recent Research Advances, Oxford & IBH Publishing Co., Pvt. Ltd., New Delhi, 2000.
- K. L. Sachidanandamurthy and H. N. Yajurvedi, “Monthly Variations of Water Quality Parameters (Physico-Chemical) of a Perennial Lake in Mysore City,” Indian Hydrobiology, Vol. 7, No. 1-2, 2004, pp. 217-228.
- D. J. Forsyth and R. H. S. McColl, “Limnology of Lake Ngahewa, North Island N.Z,” Journal of Marine and Freshwater Research, Vol. 9, No. 1, 1975, pp. 311-332.
- J. M. Melack, “Primary Productivity and Fish Yields in Tropical Lakes,” Transactions of the American Fisheries Society, Vol. 105, No. 5, 1976, pp. 575-580. doi:10.1577/1548-8659(1976)105<575:PPAFYI>2.0.CO;2
- T. R. Oglesby, “Relationship of Fish Yield to Lake Phytoplankton Standing Crop, Production and Morphoedaphic Index,” Journal of the Fisheries Research Board of Canada, Vol. 34, No. 12, 1977, pp. 2271-2279. doi:10.1139/f77-305
- G. Idodo-Umeh, “Contaminations of Heavy Metals in Water and Hemichromis fasciatus of a Waste Pit Influenced by Petroleum Activities,” Tropical Freshwater Biology, Vol. 16, No. 2, 2007, pp. 45-56.
- H. P. Stirling, “Chemical and Biological Methods of Water Analysis for Aquaculture,” University of Stirling, Stirling, 1985.
- H. I. Henderson, “The Significance of Limnology in the Development of Fisheries in Man-Made Lake and RiverBasins,” The Proceedings of the International Conference of Kainji Lake and River Basins Development in Africa, Ibadan, 11-17 December 1977.
- M. Holden and W. Reed, “West African Freshwater Fish,” Longman Group, London, 1978.
- G. E. Beedham, “Identification of the British Mollusca,” Pitman Press, Bath, 1972.
- H. Maochlan, “Illustration of Freshwater Plankton,” China Press, Kuala Lumpur, 1983.
- J. G. Needham and P. R. Needham, “A Guide to the Study of Freshwater Biology,” 5th Edition, Holden-Day, Inc., San Francisco, 1984.
- C. Y. Jeje and C. F. Fernando, “A Practical Guide to the Identification of Nigerian Zooplankton (Cladocera, Copepoda and Rotifera),” KLRI, New Bussa, 1986.
- R. A. Vollenweider, “A Manual on Methods for Measuring Primary Production in Aquatic Environments,” IPB Handbook No. 12, 3rd Edition, Oxford Blackwell, Oxford, 1985.
- R. Rump and R. Krist, “Manual for the Laboratory Examinations of Water, Wastewater and Soil,” 1987.
- T. R. Parsons, Y. Maita and C. M. Lalli, “Manual of Chemical and Biological Methods for Seawater Analysis,” Pergamon Press, Oxford, 1984.
- APHA, “Standard Methods for the Examinations of Water and Wastewater,” 19th Edition, America Public Health Association, Washington DC, 1995.
- FEPA, “Federal Environmental Protection Agency. S.1.9 National Interim Guidelines and Standards for Industrial Effluents, Gaseous Emissions and Hazardous Waste Management in Nigeria,” 1991.
- World Health Organisation, “Guidelines for Drinking Water Quality,” WHO, Geneva, 1984.
- World Health Organisation, “Environmental Health Criteria, No. 10: Principles for Safety Assessment of Food Additives and Contaminants in Food,” WHO, Geneva, 1987.
- W. Chen, S. K. Tan and J. H. Tay, “Distribution, Fractional Composition and Release of Sediment-Bound Heavy Metals in Tropical Reservoirs,” Water, Air & Soil Pollution (Historical Archive), Vol. 92, No. 3-4, 1996, pp. 273- 287.
- E. K. Ajani and S. O. Ayoola, “The Heavy Metals in the Muscle of Some Imported Frozen Fish in Ibadan, Nigeria,” Journal of Environmental Extension, Vol. 6, 2007, pp. 1-4. doi:10.4314/jext.v6i1.2753
- S. E. Kakulu and O. Osibanjo, “A Baseline Study of Mercury in Niger Delta Area of Nigeria,” Environmental Pollution, Vol. 11, No. 4, 1986, pp. 315-322. doi:10.1016/0143-148X(86)90048-0
- H. A. Hervey and C. Lee, “Hydrology and Plankton of Eleiyele Reservoir,” Hydrobiologia, Vol. 30, 1982, pp. 154-176.
- O. G. Adeyemi, G. O. Adediran and T. Oyeniyi, “Some Trace Elements Concentration in Variety of Fishes from Asa River, Ilorin, Nigeria,” Bioscience Research Communications, Vol. 8, No. 2, 1996, pp. 99-102.
- I. A. Amoo, O. T. Adebayo and A. J. Lateef, “Evaluation of Metals in Fishes, Water and Sediments of Lake Kainji,” Nigerian Journal of Food, Agriculture and Environment, Vol. 3, No. 1, 2005, pp. 209-212.
- S. N. Umeham, “Some Aspects of the Physicochemical limnology of Lake Chad (Southern Sector),” Journal of Aquatic Sciences, Vol. 4, 1989, pp. 21-26.
- A. K. Gupta and R. S. Mehrotra, “Studies on Seasonal Variation in pH and DO Content in Senthil Sarovar, Kurukshetra,” Geobios, Vol. 13, No. 6, 1986, pp. 276-278.
- L. R. Bhatt, P. Lacoul, H. D. Lekhak and P. K. Jha, “Physico-Chemical Characteristics and Phytoplankton of Taudaha Lake, Kathmandu,” Pollution Research, Vol. 18, No. 4, 1999, pp. 353-358.
- S. M. Banerjea, “Water Quality and Soil Condition of Fishponds in Some States of India in Relation to Fish Production,” India Journal of Fisheries, Vol. 14, No. 1-2, 1967, pp. 115-144.
- G. E. Hutchinson, “A Treatise on Limnology. Geography, Physics and Chemistry,” John Wiley & Sons Inc., New York, 1957.
- S. B. Saxena and A. D. Adoni, “Diurnal Variation in Sagar Lake, Sagar (India). 1. Studies in Deep Water Areas,” Hydrobiologia, Vol. 37, No. 1, 1973, pp. 7-31.
- S. Ayyappan and T. R. Gupta, “Limnology of Ramsamdra Tank-Hydrograph, Mysore,” Journal of Agricultural Science, Vol. 15, 1981, pp. 305-312.
- P. S. Welch, “Limnology,” McGraw-Hill Book Co. Inc., New York, 1952.
- W. Ohle, “Chemisch-Stratigraphische Untersuchungen der Sediment-Metamosphozeeines eines Waldsees,” Biochemische Zeitschrift, Vol. 258, 1993, pp. 420-428.
- G. L. Shroeder, “Fish Farming in Manure Loaded Ponds,” Proceedings ICLARMSEARCA Conference in Integrated Agriculture and Aquaculture Farming Systems, ICLARM Proceedings, Vol. 4, 1980, pp. 73-86.
- O. M. Lind, “Handbook of Common Methods in Limnology,” The C.V. Mosby Company, London, 1979.
- P. O. Ajah, “Identification and Mass Production of Zooplankton Species Suitable for Early Feeding of African Clariid Catfish,” Ph.D. Thesis, University of Calabar, Calabar, 1995.
- A. A. Agrawal, “Induced Responses to Herbivory in Wild Radish: Effects on Several Herbivores and Plant Fitness,” Ecology, Vol. 80, No. 5, 1999, pp. 1713-1723. doi:10.1890/0012-9658(1999)080[1713:IRTHIW]2.0.CO;2
- H. C. Kataria, “Preliminary Study of the Drinking Water of Piparian Township,” Pollution Research, Vol. 19, No. 4, 2000, pp. 645-649.
- R. C. Hart and C. Boane, “Limnology of Southern African Coastal Lakes-New Vistas from Mozambique,” African Journal of Aquatic Science, Vol. 29, No. 2, 2004, pp. 145-159. doi:10.2989/16085910409503806
- K. F. Anil and M. Musaddiq, “A Study on PhysicoChemical Characteristic of Kapshi Lake and Purna River Waters in Akola District of Maharastra (India),” Nature Environment and Pollution Technology, Vol. 1, No. 3, pp. 261-263.
- K. S. Pande and S. D. Sharma, “Studies on Water Quality Index for Ramaganga River at Moradabad, UP,” Pollution Research, Vol. 18, No. 3, 1999, pp. 327-333.
- S. Rajan, “Biology of Madras Drinking Water Supply System and Health,” Ph.D. Thesis, University of Madras, Madras, 1986.
- A. Screenivasan, “Limnology and Productivity of Tropical Upland Impoundments in Nilgiris, Madras States India,” Phytos, Vol. 7, No. 1, 1965, pp. 146-160.
- C. N. Sawyer, “Fertilization of Lake by Agriculture and Urban Drainage,” Journal of New Water Lakes Association, Vol. 51, 1947, pp. 109-127.
- I. U. Ubong and A. E. Gobo, “Fundamentals of Environmental Chemistry and Meteorology,” Tom & Harry Publications Ltd., 2004.
- S. Rajan and J. Azariah, “Seasonal Changes of Planktons in the Three Water Supply Reservoirs of Madras Drinking Water Supply, Tamilnadu—A Public Health Approach,” Indian Hydrobiology, Vol. 7, No. 1-2, 2004, pp. 205-215.
- J. M. Symons, A. A. Stevens, R. M. Clark, E. E. Geldreich, D. T. Love and J. Demarco, “Treatment Techniques for Controlling Trihalomethanes in Drinking Water,” US Environmental Protection Agency, Cineinnati, 1981.

