High-Grade Mucoepidermoid Carcinoma Ex-Pleomorphic Adenoma of the Parotid Gland: Case Report and Review of Literature ()
Keywords: Mucoepidermoid Carcinoma; Carcinoma ex-Pleomorphic Adenoma; Pleomorphic Adenoma; Parotid Gland
1. Introduction
Carcinoma ex-pleomorphic adenoma (CxPA) is an uncommon malignancy, accounting for roughly 11% of primary tumors of the salivary gland. Most primary malignant salivary gland carcinomas have been reported as the malignant component in CxPA; however, the presence of mucoepidermoid carcinoma (MEC) arising from pleomorphic adenoma (PA) has been rarely reported, with a total of nine cases in the literature.
Herein, we report a case of a high-grade mucoepidermoid carcinoma ex-pleomorphic adenoma within the deep lobe of the parotid gland.
2. Case Presentation
A 71-year-old man with a forty pack-year smoking history presented with an enlarging left neck mass over several months. Physical examination showed a firm, immobile, nontender, 3 × 3 cm left level II mass with no overlying skin change. The remainder of the head and neck examination was unremarkable.
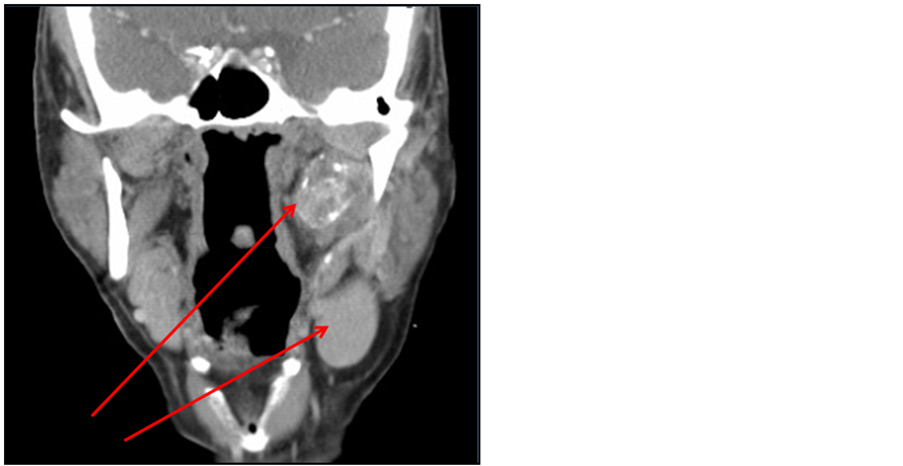
Fine needle aspiration (FNA) of the neck mass was consistent with poorly differentiated metastatic carcinoma, likely of squamous origin. Computerized tomography (CT) with contrast revealed a 3.0 × 3.0 × 1.7 cm mass within the left parapharyngeal space contiguous with the deep lobe of parotid, located between the carotid sheath and medial pterygoid muscle; a separate sharply circumscribed homogenous left level II. A lesion measuring 2.7 × 2.4 × 3.8 cm was also noted. Positron emitted tomography (PET) revealed hypermetabolic activity within both masses with no other lesions (Figure 1).
The patient underwent resection of the left parapharyngeal mass and ipsilateral selective level II - IV lymphadenectomy via transcervical-transparotid approach with facial nerve dissection and preservation. Intraoperative frozen sections were inconclusive, though histologic distinctions were seen between the parotid and cervical
 (a)
(a) (b)
(b)
Figure 1. (a) Neck CT with contrast showing left parapharyngeal space mass measuring 3.0 × 3.0 × 1.7 cm and separate, sharply circumscribed homogeneous left level IIA lymph node measuring 2.7 × 2.4 × 3.8 cm. (b) PET scan showing two areas or hypermetabolic activity within the left neck correlating with the CT.
lesions. The deep parotid lesion contained epidermoid and mucinous components whereas the neck mass solely contained an epidermoid component. Microscopic examination of the parapharyngeal mass showed a well demarcated, hyalinized fibrotic nodule adherent to parotid tissue. There were scant epithelial components with residual normal ductules among chondromyxoid stroma, epithelial cells intermingled with mucin-positive cells, and calcified areas merging with viable and necrotic tumor. The level II lymph node showed discrete nodules resembling granulomas, flanked by histiocytes with metastatic epithelial components and negative mucicarmine stain (Figure 2).
Final pathology revealed high grade mucoepidermoid carcinoma arising from a pleomorphic adenoma. The primary tumor was resected with negative margins. Although only one of thirteen nodes excised was positive, the level II node was determined to be metastatic MEC. The patient was referred to radiation oncology for intensity-modulated radiation therapy, but decided against radiation treatment for fear of side effects despite extensive communication about its post-operative indications. The patient has been compliant with serial surveillance examinations and PET scans, which have remained normal after two years.
3. Discussion
Pleomorphic adenoma (PA) is the most common salivary gland neoplasm, accounting for over 60% of parotid gland tumors. Most originate within the superficial lobe, but less frequently involve the deep lobe [1]; of these, even fewer extend medially through the stylomandibular tunnel into the prestyloid parapharyngeal space (PPS). There may be significant PPS extension before symptom onset; indeed, until the tumor is at least 2.5 cm in diameter it cannot be detected by palpation [2]. Symptoms are usually rare or insignificant, most commonly presenting asasymptomatic gradual swelling without facial nerve involvement—a nonspecific finding seen in the majority of parotid masses [3]. Rapid growth, change in consistency, pain and onset of facial nerve deficit are signs of carcinomatous transformation, the incidence of which increases with duration of a known PA [1]. Tumor recurrence, radiation therapy and advanced age are additional risk factors. Development of malignancy is estimated to occur in less than 10% of cases [3,4].
Carcinoma ex-pleomorphic adenoma is a rare, aggressive neoplasm that may present de novo or in recurrent PA, accounting for roughly 11% of all malignant salivary neoplasms. Regional metastasis is common and mortality is high [4]. Diagnosis requires histologic demonstration of both invasive adenocarcinoma, most commonly of the poorly differentiated, “not otherwise specified” variety, juxtaposed with regions of benign mixed tumor. The temporal relationship between this malignancy and its preceding lesion is complex; only a minority of patients have a previously known or treated PA [3]. Gross surgical resection and neck dissection with adjuvant radiotherapy is primary modality of treatment [1,3] though 20% - 30% of patients develop locoregional recurrence and over 30% - 40% develop metastasis. Metastasis consists of the carcinomatous element alone [4].
Although most major subtypes of salivary gland carcinoma have been reported in association with PA, particularly high-grade adenocarcinoma not otherwise specified or salivary duct carcinoma [4], the existence of MEC, the most common primary parotid malignancy in adults, has been disputed and rarely reported [5], with a mere nine total cases in the literature. Of these, six were high grade mucoepidermoid carcinoma ex-pleomorphic adenoma (MECxPA), two were low grade and one was intermediate grade [5-8]. All but one were located within the parotid gland and none were within the PPS nor metastatic at presentation. Cytologic diagnosis was misleading in 70% of reported cases (Table 1). All patients presented with an enlarging mass within the involved salivary gland. Only one patient had a history of recurrent PA.

Table 1. Clinicopathologic data on ten patients with histologic mucoepidermoid carcinoma arising from pleomorphic adenomas.
Abbreviations: N/A, not available; MECxPA, mucoepidermoid carcinoma ex-pleomorphic adenoma; Ca. NOS, carcinoma not otherwise specified; SCC, squamous cell carcinoma.
The above case describes a novel presentation of high grade MECxPA as a parapharyngeal space tumor with regional metastasis. This case proved a diagnostic challenge given the sparse literature, presentation as a metastatic neck mass, de novo occurrence within the PPS, a misleading FNA suggesting metastatic squamous cell carcinoma (SCCA) with unknown primary, and an inconclusive frozen section. Final pathology was ultimately confirmed via a positive mucicarmine stain, confirming acellular mucin and mucin-producing cells amongst epithelial cells within the parotid.
The transformation of PA in MEC may not be surprising as there is close phenotypic and ultrastructural resemblance between intermediate cells of MEC and myoepithelial cells of PA and common karyotypic alterations are found in both tumors, underscoring a link between these two entities [5].
Multiple studies on FNA specimens from salivary glands have confirmed high accuracy in distinguishing benign from malignant lesions. CxPA of the salivary gland, however, poses diagnostic difficulty on FNA [6], with some studies reporting sensitivities less than 30%; this has important clinical implications and may misdirect initial management towards incorrect treatment [3]. Similarly, only 30% (two high grade, one low grade) of the reported cases had cytologic features consistent with MECxPA, such as mucous-containing and squamous malignant cells in a chondromyxoid background with myoepithelial cells. High grade MEC was misdiagnosed, since the high grade component, although always present, was too similar to other metastatic or primary high grade malignancies such as adenocarcinoma not otherwise specified or SCCA [5]. Low grade MEC was misdiagnosed as PA, as both entities share many similar cytologic features. These malignant smears may contain sheets of epidermoid cells without cellular pleomorphism and lack mucin-containing cells, thus favoring PA, while PA may be hypercellular, contain mucin and lack characteristic chondromyxoid stroma [6]. In the presented case, FNA was only attempted on the metastatic node, since the primary lesion was incidentally found on imaging. Even so, aspiration yielded a false diagnosis of metastatic SCCA, likely due to the high grade nature of this malignancy with a predominant carcinomatous epidermoid component. In fact, in one-third of reported cases of high grade MECxPA, FNA sample yielded a false diagnosis of SCCA. These limitations, however, should not preclude the utility of FNA in initial workup of such lesions [5].
Histopathologic diagnosis requires presence of a benign mixed component adjacent to a malignant component and foci of apparent transition between benign and malignant areas may be demonstrated. This may also be challenging, as the residual mixed tumor component may be small and overlooked. In fact, up to 100 cuts may be necessary to find a small mixed tumor within a salivary gland carcinoma [4]. The malignant component may even completely replace the benign component; in such cases the diagnosis is inferred from the presence of hyaline scarring, highly characteristic of degenerated PA [3]. Furthermore, the malignant component may be difficult to classify [5], providing an additional challenge for pathologic diagnosis.
Pathology within the illustrated case is consistent with prior reports of high grade MECxPA. All tumors showed frequent mitotic figures and infiltrative patterns with adjacent areas of chondromyxoid stroma and calcification in varying degrees. The malignant component was either composed of intermediate and squamous cells with focal areas of mucous cells or sharply demarcated squamous islands with focal mucin-producing cells [5]. Microscopy of the metastatic lymph node in our case revealed presence of the carcinomatous epithelial component alone with no mucin.
Treatment of MECxPA should be similar to that of MEC. Complete surgical resection is the mainstay of treatment for all grades of MEC. The indications for lymphadenectomy and/or adjuvant radiotherapy should be individualized based on overall clinical assessment and risk of recurrence. Low-grade MEC behaves less aggressively and is typically treated with surgical excision alone. High-grade tumors are generally treated with wide surgical excision with lymphadenectomy and adjuvant radiotherapy. Intermediate-grade treatment is not well established and has varied from local excision to wide excision with lymphadenectomy and/or postoperative radiotherapy [9,10].
4. Conclusion
We present the tenth reported case of MECxPA. This case describes a rare tumor with an unlikely presentation and challenging diagnostics. It has yet to be described as a parapharyngeal mass with metastasis. As such, the pathology included herein is unique.
[2] B. Sergi, A. Limongelli, E. Scarano, A. R. Fetoni and G. Paludetti, “Giant Deep Lobe Parotid Gland Pleomorphic Adenoma Involving the Parapharyngeal Space. Report of Three Cases and Review of the Diagnostic and Therapeutic Approaches,” Acta Otorhinolaryngologica Italica, Vol. 28, No. 5, 2008, pp. 261-265.
[3] J. Rodriguez-Ciurana, C. Rodado, M. Saez and C. Bassas, “Giant Pleomorphic Adenoma Involving the Parapharyngeal Space: Report of a Case,” Journal of Oral and Maxillofacial Surgery, Vol. 58, No. 10, 2000, pp. 1184-1187. http://dx.doi.org/10.1053/joms.2000.9587
[4] S. A. R. Nouraei, K. L. Hope, C. G. Kelly, N. R. McLean and J. V. Soames, “Carcinoma ex Benign Pleomorphic Adenoma of the Parotid Gland,” Plastic and Reconstructive Surgery, Vol. 116, No. 5, 2005, pp. 1206-1213. http://dx.doi.org/10.1097/01.prs.0000181654.68120.0f
[5] K. D. Olsen and J. E. Lewis, “Carcinoma ex Pleomorphic Adenoma: A Clinicopathologic Review,” Head Neck, Vol. 23, No. 9, 2001, pp. 705-712. http://dx.doi.org/10.1002/hed.1100
[6] J. Klijanieko, A. K. El-Naggar, V. Servois, J. Rodriguez, P. Validire and P. Vielh, “Mucoepidermoid Carcinoma ex Pleomorphic Adenoma: Nonspecific Preoperative Cytologic Findings in Six Cases,” Cancer, Vol. 84, No. 4, 1998, pp. 231-234. http://dx.doi.org/10.1002/(SICI)1097-0142(19980825)84:4<231::AID-CNCR8>3.0.CO;2-P
[7] J. C. Jacobs, “Low Grade Mucoepidermoid Carcinoma ex Pleomorphic Adenoma: A Diagnostic Problem in Fine Needle Aspiration Biopsy,” Acta Cytologica, Vol. 38, No. 1, 1994, pp. 93-97.
[8] M. B. Pitman, “Mucoepidermoid Carcinoma ex Pleomorphic Adenoma of the Parotid Gland,” Acta Cytologica, Vol. 39, No. 3, 1995, pp. 604-606.
[9] M. W. Stanley and T. Lowhagen, “Mucin Production by Pleomorphic Adenomas of the Parotid Gland: A Cytologic Spectrum,” Diagnostic Cytopathology, Vol. 6, No. 1, 1990, pp. 49-52. http://dx.doi.org/10.1002/dc.2840060111
[10] M. A. Nance, R. R. Seethala, Y. Wang, et al., “Treatment and Survival Outcomes Based on Histologic Grading in Patients with Head and Neck Mucoepidermoid Carcinoma,” Cancer, Vol. 113, No. 8, 2008, pp. 2082-2089. http://dx.doi.org/10.1002/cncr.23825
[11] S. Ghosh-Laskar, V. Murthy, T. Wadasadawala, et al., “Mucoepidermoid Carcinoma of the Parotid Gland: Factors Affecting Outcome,” Head Neck, Vol. 33, No. 4, 2011, pp. 497-503. http://dx.doi.org/10.1002/hed.21477