Clinical and pharmacological properties of new oral anticoagulants for the prevention of cerebral thromboembolism: Factor Xa and thrombin inhibitors ()
1. INTRODUCTION
Stroke is a major source of mortality and long-term disability worldwide [1]. Stroke of thromboembolic origin is associated with prolonged hospitalization and a worse functional outcome [2]. Non-valvular atrial fibrillation is a major risk factor for cerebral embolism (odds ratio of 1.9 - 18.2 depending on the presence of other vascular risk factors) [3]. Patients with non-valvular atrial fibrillation have a fivefold increased risk of developing ischemic stroke [4]. Non-valvular atrial fibrillation is the most common cardiac arrhythmia, affecting 1% of the general population, and its prevalence increases with age. Atrial fibrillation can be found in approximately 10% of individuals aged 80 years and older [5]. Anticoagulant therapy is indicated in patients with atrial fibrillation and at least one moderate risk factor (e.g., hypertension, diabetes mellitus, heart failure) [6,7]. For decades, vitamin K antagonists, such as warfarin and phenprocoumon, have been the only oral anticoagulants for stroke prevention in patients at risk of cerebral embolism. Although they can decrease the risk of stroke by up to 70%, they have several important limitations. Vitamin K antagonists have a narrow therapeutic window and require regular dose adjustments by monitoring the anticoagulant effect. Other disadvantages are the risk of major intracranial and gastrointestinal bleeding complications, interactions with a number of drugs and nutrients, slow onset and offset of action and some other side effects such as coumarininduced hepatitis [8,9]. Antiplatelet drugs, such as acetylsalicylic acid and clopidogrel, were recommended for patients with atrial fibrillation who had contraindications for vitamin-K antagonists [8]. However, antiplatelet agents only provide very modest protection against cerebral embolism.
New drugs such as direct thrombin inhibitors (e.g., dabigatran), direct factor Xa inhibitors (e.g., apixaban, rivaroxaban) or indirect factor Xa inhibitors (e.g., fondaparinux, idraparinux, indrabiotaparinux) were recently developed and are currently being tested in clinical trials. Dabigatran and rivaroxaban were recently approved for the prevention of thromboembolism in patients with atrial fibrillation.
This review will focus on thrombin inhibitors and factor Xa inhibitors, which are new and promising oral anticoagulants in the most advantages stages of clinical development. We will discuss the pharmacological and clinical properties of these substances and provide the most recent updates on their clinical trials.
2. CONVENTIONAL DIRECT UND INDIRECT ANTICOAGULANTS
Coumarins, or 4-hydroxycoumarins, are the most commonly used vitamin K antagonists and, until recently, were the only oral anticoagulants available for clinical use. First introduced more than half a century ago, they are currently represented by warfarin, phenprocoumon, acenocoumarol and dicoumarol (Figure 1). In the USA, warfarin is a common oral anticoagulant, whereas in most European countries phenprocoumon is more frequently used. The precursor of these anticoagulants, coumarin, is a chemical compound found in many plants. The name coumarin is derived from a French word “coumarou” for the tonika bean (Dipteryx odorata), which has a high concentration of this compound. It has a sweet odour and has been used in perfumes and in food flavouring additives since 1882, until it was banned due to concerns about hepatotoxicity. Although coumarin itself does not show anticoagulant activity, it is transformed into the anticoagulant dicoumarol by a number of fungi. This natural anticoagulant was responsible for the cases of bleeding in cattle that ate sweet clover silage [10].
Warfarin is a synthetic derivative of coumarin and was initially used as a rodenticide before its therapeutic value was recognized. Warfarin and other pharmaceuticals derived from coumarin inhibit the enzyme vitamin K epoxide reductase, which converts vitamin K to its active form. The amount of available vitamin K in the body decreases, resulting in reduction of the activity of vitamin K-dependent enzymes involved in the transformation of certain clotting factors into their active forms [8]. Warfarin and other vitamin K antagonists have a number of disadvantages, such as diverse interactions with other drugs (e.g., antiepileptics) and nutrients, complex pharmacokinetics and pharmacodynamics, and a narrow therapeutic window. They also have potential teratogenicity and can show hepatotoxicity in up to 2% of patients [11]. However, oral anticoagulants are not usable in pregnancy.
In contrast to coumarins, heparins are indirect anticoagulants that potentiate the action of antithrombin. Unfractionated heparin (UH) is administered intravenously. It binds to plasma proteins, which can result in variable anticoagulant actions. Patients on heparin are at risk of osteoporosis and heparin-induced thrombocytopenia [12]. Low-molecular-weight heparins (LMWH) are obtained from UH by various methods of fractionation and have an average molecular weight of less than 8000 Da [12]. They are administered by once or twice daily dosing by subcutaneous injection. In comparison to UH, their coagulation parameters do not require monitoring, and they have a smaller risk of osteoporosis and a smaller risk of heparin-induced thrombocytopenia.
3. NEW ANTICOAGULANTS
The new anticoagulants are synthetic drugs that were

Figure 1. Chemical structure of coumarin, vitamin K antagonists and new anticoagulants.
recently introduced in an attempt to overcome the limitations of vitamin K antagonists and heparin. They were developed to act against one clotting factor by selective and reversible binding to the active site of enzymes participating in the coagulation cascade. Reversible inhibition of clotting factors is an important feature for lowering the risk of bleeding complications. The following classes of anticoagulants are currently the main focus of pharmacological research: direct thrombin inhibitors (e.g., dabigatran) [13], direct factor Xa inhibitors (e.g., rivaroxaban, apixaban, edoxanab) [14-16] and indirect factor Xa inhibitors (e.g., fondaparinux, idraparinux, indrabiotaparinux) [17].
Thrombin is the key enzyme at the end of the coagulation pathway. In addition, it participates in platelet activation and interacts with fibrinolysis and inflammatory processes. The direct inhibition of thrombin can affect all of its properties and, therefore, it is less predictable than the inhibition of factor Xa. However, the pleiotropic effect of thrombin inhibition may also confer advantages [18].
Factor Xa is a coagulation factor that acts at the convergence point of the intrinsic and extrinsic coagulation pathways (Figure 2). Due to its strategic location, factor Xa is an attractive target for anticoagulant agents. One molecule of factor Xa catalyses is the generation of more than 1000 thrombin molecules [19]. Factor Xa inhibitors reduce the thrombin burst in the propagation phase of the coagulation cascade. The direct inhibition of factor Xa does not reduce the activity of the existing thrombin. This action might be able to decrease the risk of bleeding, although this issue is still controversial [20].
4. DIRECT THROMBIN INHIBITORS
Dabigatran
Dabigatran is an oral, specific, reversible thrombin inhibitor (Figure 2). It is a very polar hydrophilic molecule that is not bioavailable after oral administration (Figure 1). Dabigatran is available as a prodrug, dabigatran etexilate, which increases the bioavailability to 6.5%. Dabigatran etexilate is converted by plasma esterases to its active metabolite dabigatran. The peak plasma level of dabigatran is achieved within 1 - 2 hours after oral administration and the half-life of dabigatran is about 14 - 17 hours [21]. The plasma protein binding is 35%. The predominant route of drug elimination is renal (80%). About 20% of dabigatran is metabolized in the liver with no involvement of cytochrome P450 enzymes [13]. The pharmacological profile of dabigatran in comparison to other new oral anticoagulants is shown in Table 1. There are no reported clinically relevant interactions of dabigatran with other drugs, except for amiodarone, verapamil and quinidine. In comparison to vitamin K antagonists, dabigatran does not require routine coagulation monitoring [13,22]. In comparison to ximelagatran, dabigatran does not induce heptatotoxicity, as far as is currently known.
The efficacy and safety of dabigatran for the prevention of cerebral and systemic embolism was investigated in patients with atrial fibrillation in a randomized evaluation of long-term anticoagulant therapy (RE-LY) [23]. This phase III study (n = 18, 113) was designed as a prospective, randomized, controlled parallel group, noninferiority trial. The patients with non-valvular atrial fibrillation were randomized to receive 110 mg dabigatran twice daily (bid), 150 mg dabigatran bid or warfarin

Figure 2. Targets of inhibition of different anticoagulants.
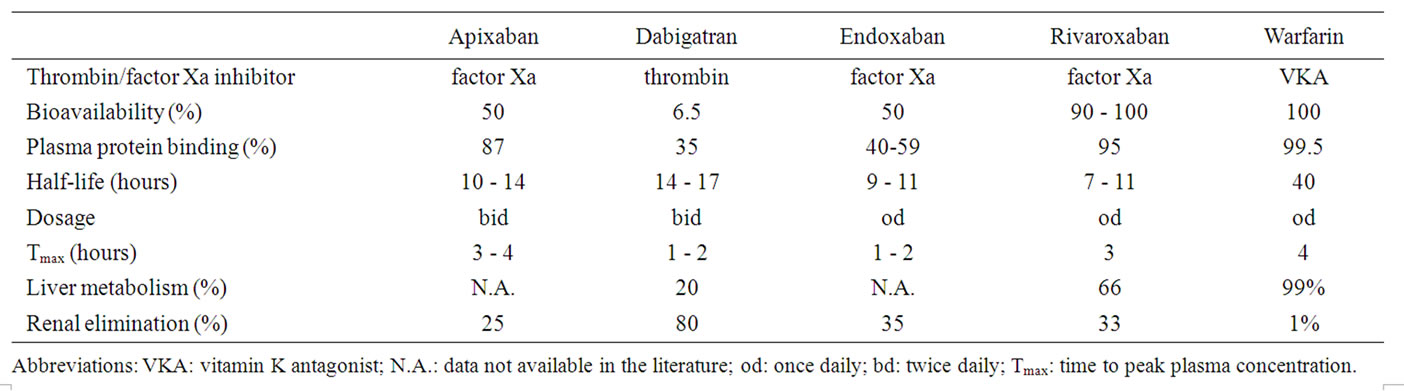
Table 1. Pharmacological profile of new oral anticoagulants compared to warfarin [13-16,20,32,33].
(INR: 2.0 - 3.0). Both doses of dabigatran were blinded, whereas warfarin was administered in an open-label manner. The annual occurrence of cerebral and systemic embolism was 1.53% in patients on 110 mg dabigatran bid, 1.11% in patients on 150 mg dabigatran bid and 1.69% in patients on warfarin. The relative risk (RR) was 0.91 (95% CI: 0.74 - 1.11) for the comparison of 110 mg dabigatran bid versus warfarin (p < 0.001 for non-inferiority and p = 0.34 for superiority) and 0.66 (95% CI: 0.53 - 0.82) for the comparison of 150 mg dabigatran bid versus warfarin (p < 0.001 for superiority). The annual rate of major bleeding complications was 3.36% in patients randomized to receive warfarin, 2.71% in patients on 110 mg dabigatran bid (p = 0.003) and 3.11% in patients on 150 mg dabigatran bid (p = 0.31). In summary, 110 mg dabigatran bid showed similar efficacy and better safety outcomes compared to warfarin (20% less major bleedings). Dabigatran at 150 mg bid was superior to warfarin in terms of efficacy outcomes (34% less embolic events) and it had a similar rate of major bleeding events as warfarin. Compared to warfarin, the relative risk of brain haemorrhage on 110 mg dabigatran bid was 69% lower and on 150 mg dabigatran bid it was 60% lower.
Table 2 shows a comparison of the number needed to treat (NNT) and the number needed to protect (NNP) for dabigatran versus warfarin and acetylsalicylate acid. The NNT of 173 for the comparison of dabigatran versus warfarin means that one cerebral or systemic embolic event could be prevented, if 173 patients changed from warfarin to dabigatran.
Based on results of the RE-LY study, dabigatran was approved for the prevention of cerebral and systemic embolism in patients with atrial fibrillation in the USA, Canada, Japan and Europe. It was also included in the last guidelines updates of the American College of Cardiology, the American Heart Association and the Heart Rhythm Society as an alternative to warfarin in the treatment of patients with atrial fibrillation [24]. A summary of the phase III clinical trials with direct thrombin inhibitors and direct factor Xa inhibitors is shown in Table 3.
Dabigatran has also been shown to be effective in the treatment of acute deep vein thrombosis and pulmonary embolism. The most frequent minor side effect of dabigatran is dyspepsia. Among disadvantages of dabigatran are the absence of antidote and the absence of routine laboratory tests for the measurement of anticoagulant effect in case of overdose. As the protein binding of dabigatran is low, it can be dialysed with the removal of approximately 60% of substance over 3 hours. In addition no data on compliance are available for dabigatran.
5. DIRECT FACTOR XA INHIBITORS
5.1. Rivaroxaban
Rivaroxaban is an oral direct factor Xa inhibitor, which has a 10,000-fold higher selectivity for factor Xa than for

Table 2. Number needed to treat (NNT) and number needed to protect (NNP)*.

Table 3. Clinical trials (phase III) of oral thrombin and factor Xa inhibitors in stroke prevention.
other serine proteases (Figures 1 and 2). The pharmacological properties of rivaroxaban are shown in Table 1. The oral bioavailability of rivaroxaban is 90% - 100%. The peak plasma level is reached in about 3 hours after digestion and the terminal half-life is 7 - 11 hours. The half-life is prolonged in the elderly and in cases of renal insufficiency [20]. About 95% binds to plasma proteins, with serum albumin representing the main binding component. Its pharmacokinetic and pharmacodynamics profiles have been proven to be predictable for daily doses of 5 to 80 mg. Rivaroxaban is metabolized in the liver (66%), with the involvement of cytochrome P450.
The efficacy of rivaroxaban has been proven for the treatment of acute and recurrent deep vein thrombosis over a period of 24 months. The safety outcomes of rivaroxaban were found to be comparable with warfarin.
In a recent randomized double-blind study of patients with atrial fibrillation, rivaroxaban (20 mg daily or 15 mg daily in creatinine clearance 30 – 49 mL/min) was compared with warfarin (INR 2-3) [25]. The mean follow-up of this study, with approximately 7130 patients per treatment group, was 12 months and the mean CHAD2 score was 3.5 in each group. In patients on rivaroxaban, the rate of cerebral and systemic embolic events was 1.7% per year versus 2.2% per year in the patients on warfarin (hazard ratio (HR) 0.79, 95% CI: 0.66 - 0.96, non-inferiority p < 0.001). In the intentionto-treat analysis the annual rate of stroke or systemic embolism was 2.1% under rivaroxaban and 2.4% under warfarin (OR: 0.88, 95% CI: 0.74 - 1.03, p < 0.001 for non-inferiority and p = 0.12 for superiority). The rate of major bleeding complications did not differ between warfarin and rivaroxaban (p = 0.44). Intracerebral haemorrhages occurred less frequently in patients with rivaroxaban than in patients with warfarin (0.5% versus 0.7% per year, HR 0.59, 95% CI: 0.37 - 0.93, p = 0.024).
Currently, rivaroxaban is also being investigated in the treatment of acute pulmonary embolism and in the prevention of ischaemic events in unstable angina pectoris (phase III trial) [26].
5.2. Apixaban
Apixaban is a selective and reversible inhibitor of factor Xa and pro-thrombinase activity [14-16]. It has an oral bioavailability of 50% and is rapidly absorbed with a peak plasma level being achieved 3 - 4 hours after intake. The mean half-life was found to range between 10 and 14 hours [14]. Apixaban has various pathways of elimination, such via the kidneys, the intestinal route and oxidative metabolism. Cytochrome P450 inhibitors can increase the plasma level of apixaban. Other pharmacological characteristics of apixaban are shown in Table 1.
Apixaban for the prevention of cerebral and systemic embolism was investigated in two ongoing, randomized double-blind studies. The Stroke and Other Thromboembolic Events in Atrial Fibrillation (ARISTOTLE) study randomized over 18,000 patients with atrial fibrillation to receive apixaban (5 mg bid) or warfarin (INR 2.0 - 3.0) [27]. The primary objective is the non-inferiority of apixaban regarding the risk reduction of stroke and systemic embolism. The annual rate of stroke and systemic embolism was 1.27% in patients on apixaban and 1.60% in patients on warfarin (OR 0.79, 95% CI: 0.66 - 0.95, p < 0.001 for non-inferiority and p = 0.01 for superiority). Apixaban caused fewer major bleeding complications than warfarin (OR 0.69, 95% CI: 0.60 - 0.80, p < 0.001). The annual rate of haemorrhagic stroke was 0.24% in the apixaban arm and 0.47% in the warfarin arm (OR 0.51, 95% CI: 0.35 - 0.75, p < 0.001, p < 0.001). The disadvantage of the ARISTOTLE study was that warfarin arm was not blinded.
The AVERROES study evaluated apixaban (5 mg bid) versus acetylsalicylic acid (81 - 324 mg once daily (od)) for the prevention of stroke in patients with atrial fibrillation (n = 5600) who failed on, or were unsuitable for, vitamin K antagonist treatment [28]. The annual rates of cerebral and systemic embolism were 1.6% and 3.7% in patients on apixaban and acetylsalicylic acid, respectively (HR 0.45, 95% CI: 0.32 - 0.64, p < 0.001). The rate of major bleeding complications, haemorrhagic stroke, myocardial infarction and death was similar in both treatment arms [29].
Three studies (Apixaban Dosed Orally versus Anticoagulation with Enoxoparin [ADVANCE-1, -2 and -3]) evaluated apixaban for the prevention of venous thromboembolism in patients with major orthopaedic interventions. In the ADVANCE-1 study, 2.5 mg apixaban bid failed to show non-inferiority compared to 30 mg enoxaparin (LMWH) bid regarding the primary endpoint (occurrence of deep vein thrombosis, pulmonary embolism and all-cause mortality) in patients after total knee replacement surgery [30]. The ADVANCE-2 and -3 studies showed superiority of 2.5 mg abixaban od compared to 40 mg enoxaparin od for the prevention of deep vein thrombosis, pulmonary embolism and thromboembolism-related mortality in patients who underwent total knee and total hip replacement surgery [31].
5.3. Endoxaban
Endoxaban (DU-176b) is an oral, direct, specific inhibitor of factor Xa (Figure 2). It has a bioavailability of approximately 50% and the peak plasma concentration of 60 mg orally administered endoxaban is achieved after 1.5 hours [32]. The pharmacological profile of endoxaban is shown in Table 1. Endoxaban has no clinically relevant interactions with food [33]. Data on interactions with other drugs are not currently available.
In a phase II study for the prevention of cerebral embolism in patients with atrial fibrillation, 30 mg and 60 mg endoxaban bid both showed a higher rate of major bleeding complications compared to warfarin (INR 2.0 - 3.0). Endoxaban administered once daily at a lower dose (30 mg od and 60 mg od) was associated with a similar rate of major bleeding complications and cerebral embolic events compared to warfarin. The phase III study is currently comparing 30 mg and 50 mg endoxaban od with warfarin for the prevention of cerebral and systemic embolism in nearly 20,500 patients with atrial fibrillation [34]. In fact, a number of other direct factor Xa inhibitors, such as eribaxan, betrixaban (PRT054021), LY517717, TAK-422 and YM150, are currently being investigated in phase II studies [35-40].
6. INDIRECT FACTOR XA INHIBITORS
Fondaparinux was the first synthetically generated factor Xa inhibitor (Figure 2). Similar to other heparins, fondaparinux is dependent on the presence of antithrombin. It mediates the conformational change of antithrombin, which results in a 700 times greater affinity compared to heparin [41]. Fondaparinux is approved for treatment and the prevention of venous thrombosis, pulmonary embolism and unstable angina pectoris [42]. A number of derivatives of fondaparinux, such as idraparinux, idrabiotaparinux and semiloparin, are currently under development [17].
7. CONCLUSION
Direct thrombin inhibitors and direct factor Xa inhibitors have shown promising results in clinical trials of stroke prevention to become valuable alternatives to vitamin K antagonists in the near future. The introduction of these innovative substances to the market is an important milestone in the development of safe and patient-oriented strategies for oral anticoagulation. For decades, vitamin K antagonists were the only therapeutic option for oral anticoagulation in patients with atrial fibrillation and an increased risk of cerebral embolism. Due to a number of disadvantages of vitamin K antagonists, such as routine coagulation monitoring, interactions with food and a number of drugs and bleeding complications, there is a need for new substances with a more favourable pharmacological profile. At present, the best evidence is available for dabigatran and rivaroxaban. Only a few months after approval of dabigatran by the FDA, it has entered the US guidelines of cardiology as an alternative to warfarin for preventing cerebral and systemic thromboembolism in patients with atrial fibrillation. Recently, rivaroxaban was also approved for the prevention of embolism in atrial fibrillation. In the near future, the approval of apixaban and endoxaban for oral anticoagulation in atrial fibrillation is expected. The development of new therapeutics is extremely important in the treatment of thromboembolic strokes, which often result in severe functional disability and neurological sequelae, such as post-stroke epilepsy [43] or post-stroke dementia [44].
Although many thousands of patients have already been treated with new thrombin inhibitors and factor Xa inhibitors, these new oral anticoagulants need to be further investigated in post-approval studies in order to evaluate treatment adherence in “real-life” settings and the safety aspects in different subgroups of patients. In addition, limitations of studies with new oral anticoagulants include the fact that warfarin arm was not blinded and a substantial proportion of patients did not achieve therapeutical range of INR 2 - 3. Also, the question of antagonistic treatment options in the case of bleeding complications has to be addressed.
NOTES