1. Introduction
Pond, a stagnant water body is widely used for drinking, bathing, washing for industrial and agricultural purposes. The bottom of the water reservoir is made up of sediment (i.e. sand, silt, and clay). The ponds gain water through rainfall, run-off or tail water from irrigation. However, in an urban area, the pond is polluted due to several anthropogenic activities i.e. industrial and sewage effluents, runoff water, vehicular emissions, etc. Coal, a naturally occurring combustible material is widely used for energy generation in an urban area by emitting various gasses, the inorganics and organics into the ecosystem [1] [2] [3] [4] . The complex environmental issues i.e. acid mine drainage, deposition of toxic compounds, air pollution, halting of acid rain, health hazards, storage of solid waste, etc., were arisen due to huge coal burnings [5] - [13] . The toxic chemicals i.e. fluoride, heavy metals and polycyclic aromatic hydrocarbons, etc., deteriorate the water reservoirs in various region of the World [14] [15] [16] [17] [18] . Hence, in this work, the contamination of pond reservoirs (i.e. water and sediment) with fluoride, sulfur, nutrients and heavy metals in the Korba basin, India is described.
2. Materials and Methods
2.1. Study Area
The largest coal deposits in the country are present in the Korba basin (22.35˚N and 82.68˚E). Several open and underground coal mines are in operation with annual production of ≈3 BT coal. A huge amount of coal >10,000 MT annually is consumed by the various unit of thermal power plants running in the Korba area by emitting several million tons of fly ash into the environment. The Asia’s biggest Aluminum plant is also in the operation in this area. The environment of Korba city has been polluted due to the huge exploitation of coals. The large population (≈0.5 million) residing in the basin is exposed from various environmental contaminants related to coal burning and leaching.
2.2. Sample Collection and Preparation
The water and sediment samples were collected from 26 ponds, lie over ≈500 km2 area of the Husdo river basin in May 2012 as shown in Figure 1. The cleaned one- liter narrow-mouth polyethylene bottle was used for the water collection. The container was rinsed twice, and completely filled with the sample water. The physical parameters i.e. pH, dissolved oxygen (DO) and electrical conductivity (EC) were measured at the spot by using HANNA made sensors. The sample was transferred to the laboratory and divided into two portions. The 1st portion was treated with few drops of ultra-pure nitric acid (E. Merck) for the metal analysis. The 2nd portion was used for monitoring of anions. All samples were refrigerated at 4˚C.
One kilogram of the top sediments (0 - 10 cm) was sampled by a stainless steel spoon, and stored in glass jar [19] . The sediment samples were dried, milled and sieved out particles of ≤0.1 mm for the X-ray spectroscopic analysis of the major elements. A 0.25 g of the sample was digested with acids (3 mL HCl and 1 mL HNO3) in the closed system with P/T MARS CEM (Varian Company) microwave oven. The acid extract was used for monitoring of the metals with the spectroscopic techniques.
A weighed amount of sediment sample (0.25 g) was placed in a 50 mL Pt-cru- cible by adding 2.0 g NaOH [20] . The crucible was kept in a muffle furnace and slowly raising the temperature up to 600˚C. The sample was fused up to 30 min, and the residue was dissolved in hot water. The pH of the extract was adjusted to 9.0 to precipitate the interfering ions i.e. Fe, Al, Mn. Then, it was filtered and diluted to 100 mL in a polyethylene volumetric flask for the F- analysis. The sediment sample was mixed with pure water into 1:2 (m/v) in a 100-mL conical flask. The suspension was allowed to stand for overnight, and the pH
![]()
Figure 1. Representation of sampling locations in Korba basin.
value of the settled aqueous solution was measured by a Hanna pH meter (type- HI991300).
2.3. Analysis
The physical parameters (i.e. pH, EC and DO) of the water samples were measured with the Hanna made sensors.
The Dionex chromatography DX120 equipped with anion separation column (AS9-HC, 250 × 4 mm), cation separation column (CS12A, 250 × 4 mm) and con- ductivity detector was used for analysis of the ions (i.e. Na+, K+, 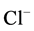 ,
, 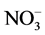 ,
, 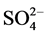 , Mg2+ and Ca2+).
, Mg2+ and Ca2+).
The F− content was analyzed by using Metrohm-720 ion meter using the fluoride selective electrode. The calibration curve was prepared by using 1.0, 3.0, 5.0, 7.0 and 10.0 mg/L F− containing the buffer solution in 1:1 ratio (v/v). The buffer was prepared by dissolving sodium citrate (300 g), 1,2-cyclohexanedia-mine-N- tetraaceticacid (22 g) and NaCl (60 g) in a volume of one liter with the de-ioni- zed water by subsequent adjustment of pH value to 5.2 ± 0.2. Ten milliliters of water sample was mixed with the buffer in a 1:1 ratio (v/v), and F− content were analyzed by using standard calibration curve.
The CHNSO-IRMS Analyzer by SV Instruments Analytical Pvt. Ltd. was used for analysis of black or elemental carbon (BC). The sediment sample (15 mg) was oxidized with O2 at 1020˚C with constant helium flow by detecting the resulting CO2 gas with a thermal conductivity detector. The H3PO4 (10 drops) treated sediment sample was oxidized in a similar way for determination of BC and OC content. The OC content was analyzed by titration method using K2Cr2O7 as oxidant [21] . The CC content in the sediment was evaluated by subtracting sum of the BC and OC content to the TC (total carbon) by using the following equation.
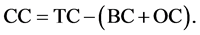
The Hitachi High-Tech Scanning electron microscope (SEM)-SU6600 equipped with the energy dispersive X-ray spectrometer (EDS) and cathode luminescence (CL) detector was used for characterization of elements (i.e. C, O, S, Cl, Na, Mg, Al, Si, P, K, Ca, Ti, Fe, Mn, and Ni). The sample was irritated with X-ray in a polyethylene disc in both secondary electron imaging (SEI) and backscattered electron imaging (BSEI) modes to record surface photographs and elemental peaks as shown in Figure 2 and Figure 3. Three measurements at different locations of a sample were carried out, and their mean values are presented.
The Varian ICP-OES-700-ES was used for monitoring of metals (i.e. Cr, Cu, Zn, and Pb) in the sediment extract. The GF-AAS SpectrAA 220 Zeeman and CV- AAS SpectrAA 55B were used for the analysis of As, Cd, and Hg. The results are expressed on a dry-weight basis. The NCS DC 73,382˚C RM sediment sample was used for the quality control. The accuracy of analysis of the metals in the reference sample was found within ≤2%. The precision (RSD) of elemental analysis (n = 3) in the sediment with the SEM-ESD, ICP-AES, AAS, ion selective and IC technique was found to be ≤12, 7, 6, 5 and 5%, respectively.
2.4. Enrichment of Contaminants
The pollution indices i.e. enrichment factor (Ef), contamination factor (Cf) and
pollution load index (PLI) were used to determine element concentration in the sediment samples with respect to the baseline concentration. The concentration ratio of an element, X to Al in the sediment was normalized to the ratio of element, X and Al present in the earth’s crust. The following equations were used for computation of the pollution indices [22] [23] .
![]()
![]()
![]()
where, Xs and Als are concentrations of metal and Al in the sediment, and Ale and Xe are background concentration of metal and Al in the earth crust.
3. Results and Discussion
3.1. Water Contamination
The geographical characteristics of twenty six ponds are summarized in Table 1.
![]()
Figure 3. EDX signals of elements in sample no. S4.
![]()
Table 1. Physio-chemical characteristic of pond water.
PN = Pragati Nagar, HTPP = Hydro Thermal Power Plant, SN = Shanti Nagar.
The catchment areas of the ponds were ranged from 1000 - 36,000 m2. Among them, five ponds exhibited with larger catchment area, ranging from 18,000 to 36,000 m2. The pH, DO and EC value of the water (n = 26) was ranged from 5.7 - 8.5, 5.8 - 8.4 mg/L and 278 - 610 µS/cm with mean value of 7.0 ± 0.3, 7.2 ± 0.3 mg/L and 423 ± 35 µS/cm, respectively. The ratio of sum of total concentration of anions to cations, Σanion/Σcation was found to be 0.8 ± 0.6. The concentration of elements i.e. F−, Cl−, ![]() ,
, ![]() , Na+, K+, Mg2+, Ca2+, Al, and Fe was ranged from 1.7 - 4.9, 12 - 46, 19 - 52, 17 - 70, 6.0 - 30, 4.0 - 19, 5.0 - 16, 16 - 45, 1.1 - 2.4 and 0.4 - 2.3 mg/L with mean value (p = 0.05) of 2.8 ± 0.4, 27 ± 4, 34 ± 4, 39 ± 6, 16 ± 2, 8.6 ± 1.4, 10.4 ± 1.2, 30 ± 3, 1.7 ± 0.1 and 1.2 ± 0.2 mg/L, respectively, Table 2. The heavy metals i.e. As, Cr, Cu, Zn, Cd, Pb and Hg in the surface water was found at microgram levels, ranging from 8 - 30, 10 - 21, 11 - 42, 76 - 300, 2 - 15, 12 - 43 and 0.6 - 3.8 µg/L with mean value (p = 0.05) of 17.2 ± 2.1, 14.0 ± 1.2, 17.2 ± 2.8, 161 ± 3, 6.7 ± 1.3, 23.4 ± 3.5 and 1.7 ± 0.4 µg/L, respectively, Table 3. Among 17 elements detected in the water, the highest concentration of
, Na+, K+, Mg2+, Ca2+, Al, and Fe was ranged from 1.7 - 4.9, 12 - 46, 19 - 52, 17 - 70, 6.0 - 30, 4.0 - 19, 5.0 - 16, 16 - 45, 1.1 - 2.4 and 0.4 - 2.3 mg/L with mean value (p = 0.05) of 2.8 ± 0.4, 27 ± 4, 34 ± 4, 39 ± 6, 16 ± 2, 8.6 ± 1.4, 10.4 ± 1.2, 30 ± 3, 1.7 ± 0.1 and 1.2 ± 0.2 mg/L, respectively, Table 2. The heavy metals i.e. As, Cr, Cu, Zn, Cd, Pb and Hg in the surface water was found at microgram levels, ranging from 8 - 30, 10 - 21, 11 - 42, 76 - 300, 2 - 15, 12 - 43 and 0.6 - 3.8 µg/L with mean value (p = 0.05) of 17.2 ± 2.1, 14.0 ± 1.2, 17.2 ± 2.8, 161 ± 3, 6.7 ± 1.3, 23.4 ± 3.5 and 1.7 ± 0.4 µg/L, respectively, Table 3. Among 17 elements detected in the water, the highest concentration of ![]() was observed due to burning and mining of the coal. They were found to
was observed due to burning and mining of the coal. They were found to
![]()
Table 2. Concentration of ion and metal in water, mg/L.
![]()
Table 3. Concentration of heavy metal in water, µg/L.
occur in following increasing order in the ecosystem: Hg < Cd < Cr < Cu < As < Pb < Zn < Fe < Al < F− < K+ < Mg2+ < Na+ < Cl− < Ca2+ < ![]() <
<![]() . The concentration of F−, As, Cr, Cu, Zn, Cd, Pb, and Hg in the surface water of the study area was found to be comparable to the values reported in other regions of the country and World [24] - [33] . The value of physical parameters i.e. pH, DO and EC of the surface water was found within recommended limits reported for drinking water [34] [35] . The tolerance limit reported for elements i.e. F−, Al, Fe, Cd, Pb and Hg in drinking water is 1.5, 0.05, 0.300, 0.005, 0.01 and 0.001 mg/L [34] [35] . The higher concentration of elements i.e. F−, Al, Fe, Pb and Hg than permissible limits was observed in all locations, mainly due to their discharge of the industrial effluent of the Aluminum and Thermal power plants. The surface water is widely used for drinking purpose by domestic animals i.e. cattle, buffalo, sheep, and coats. The reflection of fluoride toxicities in the domestic animals (i.e. cattle, buffalo, sheep, and goat) was marked as dental and bone fluorosis as shown in Figure 4.
. The concentration of F−, As, Cr, Cu, Zn, Cd, Pb, and Hg in the surface water of the study area was found to be comparable to the values reported in other regions of the country and World [24] - [33] . The value of physical parameters i.e. pH, DO and EC of the surface water was found within recommended limits reported for drinking water [34] [35] . The tolerance limit reported for elements i.e. F−, Al, Fe, Cd, Pb and Hg in drinking water is 1.5, 0.05, 0.300, 0.005, 0.01 and 0.001 mg/L [34] [35] . The higher concentration of elements i.e. F−, Al, Fe, Pb and Hg than permissible limits was observed in all locations, mainly due to their discharge of the industrial effluent of the Aluminum and Thermal power plants. The surface water is widely used for drinking purpose by domestic animals i.e. cattle, buffalo, sheep, and coats. The reflection of fluoride toxicities in the domestic animals (i.e. cattle, buffalo, sheep, and goat) was marked as dental and bone fluorosis as shown in Figure 4.
3.2. Sediment Contamination
Sediment is composed of organic and inorganic particles of various sizes. The se- diment includes boulders, cobbles, pebbles, sand, silt, and clay. The particle sizes of all sediments were found in inhomogeneous orders (Figure 2). The colour was varied from white (W) to black (B) due to deposition of the BC and fly ash particulates. Among them, 9 samples i.e. (10, 12 - 18 and 24) were contaminated with elevated levels of ash particles.
The pH value of sediments was found to be slightly acidic, ranging from 5.4 - 8.1 with mean value (p = 0.05) of 6.60 ± 0.03 due to high S content (Table 4).
The concentration of five elements i.e. C, P, O, S, and Cl in the sediments are summarized in Table 4. The concentration of TC, BC, P, O, S, and Cl was ranged from 8.9 - 31.5, 3.6 - 14.0, 0.20 - 0.92, 35.7 - 56.1, 0.08 - 1.10 and 0.11% - 0.58% with mean value (p = 0.05) of 20.9 ± 2.0, 9.2 ± 1.0, 0.46 ± 0.07, 45.9 ± 2.1, 0.57 ± 0.09 and 0.32% ± 0.05%, respectively.
The concentration of crustal elements i.e. Si, Al, and Fe was ranged from 6.0 - 14.9, 4.3 - 11.0 and 0.8% - 4.9% with mean value (p = 0.05) of 11.0 ± 0.9, 7.4 ± 0.7 and 2.1% ± 0.4%, respectively, Table 5. The concentration of metals i.e. Na, K, Mg, Ca, and Ti were occurred at moderate levels, ranging from 0.30 - 1.2, 0.37 - 1.96, 0.12 - 0.59, 0.31 - 0.88 and 0.16% - 0.88% with mean value (p = 0.05) of 0.72 ± 0.10, 0.72 ± 0.15, 0.30 ± 0.04, 0.28 ± 0.06 and 0.34% ± 0.04%, respectively, Table 5.
The heavy metals i.e. As, Cr, Cu, Zn, Cd, Pb and Hg were found to be present at the trace levels, ranging from 36 - 154, 29 - 79, 18 - 92, 42 - 294, 0.14 - 1.19, 26 - 127 and 0.12 - 0.82 mg/kg with mean value (p = 0.05) of 95 ± 12, 47 ± 5, 49 ± 8, 133 ± 28, 0.62 ± 0.11, 75 ± 13 and 0.35 ± 0.08 mg/kg, respectively, Table 6.
Among 22 detected elements, oxygen was found to exist at the highest level with the lowest value for Hg. They were occurred in following increasing trend in sediment: Hg < Cd << Cr ≈ Cu < Pb < As < Zn << Ti ≈ Mg ≈ Cl < P < S ≈ Ca < Na < K ≈ F << Fe << Al < BC < Si < TC < O. The content of three elements i.e. P, S, and Cl was observed to be much higher than the baseline value of 0.065,
![]()
Figure 4. Dental fluorosis in the buffalo.
![]()
Table 4. Physico-chemical characteristics of sediment.
0.006% and 0.037%, respectively [36] . The content of BC, F−, Cl−, As, Pb, and Hg content in the sediment of the study area was found to be higher than the values reported in the sediments of other regions of the country and World due to huge coal burning [37] - [49] .
The background concentration of Al, Fe, Na, K, Mg, Ca, P, S, F, Cl, Cr, Zn, Cu, Pb, As, Cd, and Hg reported in the earth crust was 81,530, 39,200, 24,300, 23,200, 14,900, 25,600, 650, 62, 557, 370, 92, 67, 28, 17, 4.8, 0.09, and 0.05 mg/kg, respectively [50] . The mean Ef value for Cu, Zn, Pb, Cd, P, Cl, Hg, F, As and S was computed and found to be 2.1, 2.5, 5.0, 7.8, 8.3, 10.2, 10.5, 21, 23 and 105, respectively. Three elements i.e. F−, As, and S were highly enriched, (Ef > 20) in the sediment. Five elements i.e. P, Cl, Cd, Pb, and Hg were enriched significantly, (5 ≥ Ef < 20). Whereas, other two metals: Cu and Zn were enriched moderately, (2 ≥ Ef < 5).
![]()
Table 5. Concentration of major element n sediment, %.
The Cf value for Cu, Zn, Pb, Cd, P, Cl, Hg, F, As, and S were found to be 1.7, 2.1, 4.2, 6.5, 7, 9, 9, 17, 19, and 90, respectively. The sediment was highly (Cf ≥ 6) contaminated with seven elements i.e. Cd, P, Cl, Hg, F, As, and S [51] . The sediment was contaminated moderately to significantly with three metals i.e. Cu, Zn and Pb. The PLI value for elements i.e. F, Cl, P, S, As, Pb, Cd, and Hg were found to be 16.6, 8.1, 6.6, 79, 18, 3.8, 5.7 and 7.6, respectively. These data confirmed the extreme contamination of the sediment with elements i.e. F, Cl, P, S, As, Pb, Cd, and Hg.
A good correlation (r = 0.91 - 0.99) between water and sediment content of species i.e. Cl−, As, Na, K, Ca, Cr, Zn, Cd, and Pb were observed, indicating origin from the similar sources. Poor to fair correlation (r = 0.34 - 0.72) between water and sediment content of species i.e.![]() , Mg, Al, Fe, Cu and Hg were marked, indicating contamination by multiple sources. However, a negative correlation (r = −0.28) of the F− contents of the water and sediment was seen, showing origin entirely from
, Mg, Al, Fe, Cu and Hg were marked, indicating contamination by multiple sources. However, a negative correlation (r = −0.28) of the F− contents of the water and sediment was seen, showing origin entirely from
![]()
Table 6. Concentration of heavy metals in sediment, mg/kg.
different sources. A good correlation (r = 0.97) of F− with Al content of the sediment was observed, indicating origin mainly from the Aluminum plant effluents. A fair correlation (r = 0.42 - 0.84) of the BC with the elements i.e. As, Fe, Cu, Zn, Pb, Pb, and Hg in the sediment was marked, indicating the origin mainly from the coal-burning processes, Table 7. A fair correlation (r = 0.29 - 0.88) of these elements in the sediment among themselves was recorded, showing origin from multiple sources i.e. coal burning, fly ash, runoff water, etc.
4. Conclusion
The concentration of elements i.e. F−, Al, As, Fe, Cd, Pb, and Hg in the surface wa- ter was found to be above the permissible limits. Three elements i.e. F−, S, and As were highly enriched in the sediment. The high BC content in sediment may demobilize the metal contents. However, the high fluoride fraction in the ponds was seen to reflect as fluorosis diseases in the animals. A careful evaluation of the health
![]()
Table 7. Correlation matrix of BC and heavy metals in sediment.
condition of the human being and animals using this contaminated water is urgently required.
Acknowledgements
We are thankful to Pt. Ravishankar Shukla University, Raipur, India for awarding scholarship to one of the authors: R. S.