Electrolytic Removal of Cadmium, Lead and Copper from Wastewater ()
Received 11 March 2016; accepted 15 April 2016; published 18 April 2016

1. Introduction
Toxic metals such as lead, copper and cadmium and their respective salts have been used in several industrial segments and, consequently, have led to a series of large-scale environmental contamination in the modern world. Owing to its toxicity and bio-accumulation these have caused illness for workers in these industrial segments. Some diseases, such as lead poisoning (saturnism) is associated with contamination by lead [1] - [4] , copper is associated with Wilson’s disease [5] - [7] , while cadmium focuses on the bones and causes “itai-itai” disease, so-called after the contamination that occurred in Japan in early 1970 [8] - [10] .
Cadmium is a grey metal with a metallic shine, is soft, ductile and malleable, whose surface darkens when in contact with the air owing to the formation of an oxide layer. It is a rare element and its concentration in the earth’s crust is of the order of 0.1 - 0.5 g/kg, and is normally associated with the ores of zinc, lead and copper. Cadmium is used primarily in rechargeable batteries, pigments, in the stabilizers for PVC, metal coatings and in certain alloys or compounds [8] [11] .
More than 70% of lead is used in the manufacture of lead batteries, owing to their low price, high reliability and good performance. The remainder is used in the manufacture of alloys, insulators for X-rays, ammunition and lead salts. Effluents of these industries can affect the environment and workers dramatically. The possibility of exposure to lead can cause adverse effects on various parts of the human body. The parts most affected are the brain and the entire nervous system, kidneys, blood and the male reproductive system. Relatively low levels of pellets can affect a developing fetus and young children, impairing their mental development [12] [13] .
Copper can be considered as one of the earliest metals known to man where its applications has been present in various social and industrial areas, and its technologies involve, directly or indirectly, living beings and the environment. Copper is found naturally in rocks, soil, water, sediments and, at lower levels, in the air, and its average concentration in the earth’s crust is about 50 g/kg. World copper production exceeds 23 million tons/ year, with a large part of the copper produced from sulfide minerals found in deep deposits. However, the metallic waste from copper and its alloys from target industries, consumer electronics and solid waste (or electronic rubbish) from electrical and electronic equipment have contributed to more than 35% of the production end of copper [14] .
In Brazil this is already an issue for waste disposal and the standard for cadmium, lead and copper in effluent discharge is set by the CONAMA resolution 430/2011 [15] , which sets the maximum allowed value at 0.2 mg Cd2+/L; 0.5 mg Pb2+/L and 1.0 mg Cu2+/L, respectively. Owing to the high toxicity of cadmium, lead and copper, along with their widespread use in industry, specific treatments for the removal of these elements from waste should be studied. Research should be conducted to develop new methods of metal removal or to improve existing methods.
In addition to warning the population about the evils caused by these toxic metals, this study aims to demonstrate a simple electrolytic treatment capable of removing these ions from industrial effluents efficiently, while causing minimal impact to the environment.
2. Materials and Methods
An electrochemical cell is the basis of the process, consisting of a cathode and an anode immersed in a solution, as shown in Figure 1. When a current is applied to the cell by a rectifier, the metals will be deposited on the cathode, thereby obtaining the separation. The electrochemical recovery of metals basically involves two steps:
![]()
Figure 1. Schematic assembly of removal process.
electrode position of the metal, followed by some form of stripping to remove the metal from the cathode. This stripping process can be accomplished by chemical or electrochemical dissolution, by electrolysis quarrying or reversing the polarity of the electrode.
Solutions were prepared initially containing cadmium, lead and copper ions, respectively, based on soluble salts. These simulations were used as effluent. The salt used was cadmium acetate dihydrate, lead acetate trihydrate and anhydrous copper sulfate. The concentrations used were 100, 150 and 200 mg/L for each ion.
In the experiments an electrolytic cell was used, made up of an acrylic container with a maximum capacity of 500 mL, and containing the anode (positive pole) and the cathode (negative pole) connected to a power supply of dimmable direct current (current rectifier), with a voltage of 20 - 30 V. The continuous agitation of the solution was done with a magnetic stirrer. The temperature was set at 25˚C.
The anode was made up of a thin plate of platinum, with a total area of 4.2 cm2. For the cathode, a rectangular screen of carbon steel (6.5 cm × 4.0 cm) was used, with a wire diameter of 0.30 mm and a mesh opening of 0.55 mm. Each screen had 47 wires of 6.5 cm and 77 wires of 4 cm, with a total area of 57.82 cm2. The carbon steel screen is shown in Figure 2.
Some operational conditions, such as the time and current/voltage for this experiment, were based on Faraday’s law (Equation (1)). This equation requires that: a) the amount of substance deposited is directly proportional to the amount of electricity passed through the electrolytic solution; and b) the quantities of different substances are deposited in proportion to their electrochemical equivalents. Thus, using Equation (1) [16] [17] , it is possible estimate the time required to deposit the metal on the carbon steel cathode.
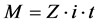 (1)
(1)
where:
m = mass, g;
Z = electrochemical equivalent (g/coulomb);
i = current, A;
t = time, h.
Using this equation for copper, lead and cadmium ions, it is possible to calculate the theoretical amount of metal that can be deposited on the cathode in an experiment. As the intention was to remove all of these ions from the various solutions in this way, under predetermined conditions of operation, it is possible choose the variable of interest over time.
After preforming the removal experiments, the residual concentrations of Cd2+, Cu2+ and Pb2+ ions in each sample were checked using a voltammetric analyzer (VA 767 Metrohm Computrace). This equipment works with three electrodes combined: the working electrode (mercury multimode), reference electrode (Ag/AgCl-KCl 3.0 mole/L) and auxiliary platinum electrode. The removal efficiencies and average velocity removal were then calculated using Equation (2) and Equation (3), respectively,
![]()
Figure 2. Carbon steel screen used as cathode.
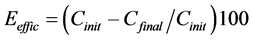 (2)
(2)
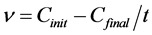 (3)
(3)
where:
Eeffic = efficiency of removal (%);
n = Average velocity removal (mg/L×min);
Cinit = concentration of solution before the electrochemical removal process;
Cfinal = concentration of the solution after the electrochemical removal process;
t = temp of removal of contaminant.
3. Results and Discussion
All experiments were repeated three times, and the results of such tests are presented on the basis of the arithmetic average.
The graph in Figure 3 shows the removal efficiency (%) applied to the Cu2+, Cd2+ and Pb2+ ions in the initial concentrations of 100, 150 and 200 mg/L, using a voltage of 20 V and a current of 0.2 A.
For cadmium, removal efficiencies (%) decreased with the increase in initial concentrations. For lead removal, efficiency was practically constant. However, for copper, there was a significant increase in removal efficiencies with increasing initial concentration.
The graph in Figure 4 shows the variation of removal efficiency for the three ions, depending on the theoretical times of deposition based on Faraday’s equation (Equation (1)). In this experiment the concentration of 100 mg/L for the Cu2+, Cd2+ and Pb2+ ions was fixed using a fixed voltage of 20 V and a current of 0.2 A. In the graph “t” is the theoretical value, while “t1” and “t2” are an increase in the time of 25% and 50% for each metal, respectively, represented by “t1 = 1.25t” and “t2 = 1.5t”.
The results show that the increase in deposition time favors the greatest removal with values greater than 90%. In these trials removal efficiencies of 94.07% for cadmium, 94.71% for lead and 96.19% for copper were obtained. For copper and cadmium increased time favored a higher percentage yield, but for lead there was a significant gain.
The graph in Figure 5 shows the variation of average velocity of removal of the three ions, fixing the concentration in 100 mg/L to the Cu2+, Cd2+ and Pb2+ ions and using a voltage of 20, 25 e 30 V. It was observed that the increase in voltage favors an increase in the deposition velocity of copper and cadmium. However, for the lead this remains almost constant. Probably this fact is a result of the conditioning properties of lead in relation to conductivity and the highest evolution of hydrogen [14] [15] .
Another issue that must be stressed is the evolution of hydrogen during the process. This was constant and probably decisive in decreasing the efficiency. Agitation was applied to minimize the consequences of this evolution.
4. Conclusions
Based on the literature and laboratory tests, the following conclusions are made:
![]()
Figure 3. Removal efficiency (%) applied to the Cu2+, Cd2+ and Pb2+ ions at 20V and 0.2 A.
![]()
Figure 4. Variation of removal efficiency (%) of three ions depending on the theoretical times of deposition at 20 V and 0.2 A.
![]()
Figure 5. Variation of average velocity of removal of Cu2+, Pb2+ and Cd2+ ions in concentration of 100 mg/L.
Electrochemistry is an effective technique for removing these metals from waste water, as its main advantages compared to conventional processes means that there is no need for chemical product input and sludge generation.
The increased reaction time resulted in an increase in the removal efficiencies, with the largest removals observed at 50% greater reaction times than theoretical times (t2 = 1.5t). In these experiments removal efficiencies of 94.07% for cadmium, 94.71% for lead and 96.19% for copper were obtained
NOTES
![]()
*Corresponding author.