Spectroscopic Analysis and Study of Charge Transport Properties for Pinacyanol Chloride-Organic Acceptor Complex as Potential Optoelectronics Material ()
1. Introduction
The subject of charge transfer complexes has been very old [1] - [3] . After finding organic metals and other quasi- one-dimensional conductors [4] [5] , this important branch of chemical physics has undergone a renewed interest. To some of the experimental scientists and applied physicists, it is known as molecular electronics. The use of CT complexes involving both organic and organometallic conductors in dry cells (batteries), p-n junction diodes, FETs, Schottkey diodes and optoelectronic devices like photodiodes, phototransistors, infrared detectors, solar cells, organic LEDs, etc. has been attempted. This is how the subject of one-dimensional conductors has been connected with advanced energetics [6] -[20] .
In the present work, we have reported transport properties and effect on band-gap of pinacyanol Chloride when it forms CT with organic acceptors. Transmission spectra of the charge transfer complexes of pinacyanol is reported. The structures of pinacyanol chloride and organic acceptors used in the present work are shown (Figure 1).
2. Experimental Details
Pinacyanol chloride―a pure spectroscopy grade was obtained from Aldrich chemical company. It was divided in four equal parts and each part was mixed with similarly pure organic acceptors, namely chloranil, DDQ (2,3- dichloro-5,6-dicyano-p-benzoquinone), TCNE (tetracyano ethylene) and TCNQ (7,7,8,8-tetracyano-p-quino- dimethane) in 1:1 molecular proportions and grinding was carried for each mixture in a black agate mortar with black agate pestle till the color deepened or changed remarkably.
The charge transfer complexes prepared as above were mixed with dry spectro-grade KBr powder in 5:95 percentage proportions. The 95% of the KBr powder led to semitransparent (not completely opaque) pellets when the charge prepared by homogenous dispersion was pressed in a die by a manually operated compressing machine. The pellets were fixed in a dark chamber of the Perkin-Elmer spectrophotometer for recording the spectra in the full infrared range, i.e. in a range 400 - 4000 cm−1.
3. Results and Interpretation
The infrared spectrum of pinacyanol chloride is shown (Figure 2(a)).
The noise which is a peculiar characteristic of a photoconducting medium [21] [22] is clearly observed in electrical conductivity and absorption coefficient in the ranges 3400 cm−1 to 4000 cm−1 and 1650 cm−1 to 2000 cm−1. This is because α and σ (conductivity) are related by,
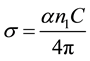
The higher range noise is due to strong coulomb repulsion among the charge carriers in conduction band. The lower range noise is due to localization near the band edges [23] -[25] .
In the mid-frequency range between 1600 cm−1 and 950 cm−1 a background triangular distribution is observed.
![]()
Figure 1. Molecular structures of pinacyanol chloride and organic acceptors.
The filled density of states for charge carrier tracks a potential barrier. If this barrier is a rectangular barrier, there is very limited transport of charge carriers because the charge carriers need threshold energy for over-barrier hopping. But when such a barrier is triangular due to interaction between chlorine and pinacyanol cation, there is tunneling probability increasing the number of transported charge carriers by three orders of magnitude. Actually this is the origin of photoconductivity of pinacyanol chloride, i.e.  as observed earlier.
as observed earlier.
There is slight increase in transmission below 1000 cm−1 and a plateau is seen below 900 cm−1. At still lower wave numbers below 750 cm−1, a weak asymmetric Gaussian envelope is observed extending down to 400 cm−1 (Figure 2(b)).
This Gaussian band is due to the coupling of rocking, wagging and group vibrations with electronic motion. The band is a characteristic of pinacyanol cation since it is observed in all the CT complexes studied in the present work. The nature of interband transition is studied in the range between 1650 cm−1 and 2000 cm−1 by plotting all possible absorption functions of direct and indirect transitions and deciding the best fit. The comparison of all graphs lead to conclude that the IR band gap is about 0.25 eV and the transition is direct allowed type. Although pinacyanol is a macro molecular substance, the transition is found to be direct rather than indirect because of the molecular symmetry. This shows that a symmetric macromolecule behaves like a small purely organic molecule having negligible dipole moment. The plot of analysis is shown (Figure 2(c)).
The infrared spectrum of (PC-Cl)-chloranil is shown (Figure 3(a)).
Again noise is observed in 3600 - 3900 cm−1 range. The lower frequency range is spread over a long range compared to PC-Cl above Eg. This is due to disordered molecules of chloranil as compared to fixed lattice of PC-Cl. There are red-shifts of many bands above Eg and blue-shifts of many bands below Eg. This shows that there is softening of high frequency vibrations above Eg and anharmonicity in low frequency vibrations below Eg. The interband transition is allowed direct with a slight lowering of the band gap (Eg − 0.22 eV) (Figure 3(b)).
![]() (a)
(a)![]() (b)
(b)![]() (c)
(c)
Figure 2. (a) IR spectrum of pinacyanol chloride; (b) Nature of transition; (c) Low-frequency gaussian.
![]() (a)
(a)![]() (b)
(b)![]() (c)
(c)
Figure 3. (a) IR Spectrum of (PC-CL)-chloranil; (b) Nature of transition in (PC-CL)-chloranil; (c) Analysis of gaussian band at low-frequency.
The mid-IR envelope centered around 1150 cm−1 is somewhat distorted from the triangular distribution and has a low-frequency tail. The shape of this envelope can be attributed to imperfect nesting or partial screening. On the other hand, the shape below 1100 cm−1 can be visualized as inverted parabola in transmission, a parabola in absorption coefficient. This can be directly related with band structure. The absorption coefficient and the density of states of charge carriers are proportional. The low-frequency Gaussian curve is preserved but there are no frequency shifts of the bands which provide fine structure. The Gaussian band is almost symmetric. It is more symmetric than same found in PC-Cl alone (Figure 3(c)).
Actually pinacyanol chloride is asymmetric due to Clv attached to the substituent chain on one side as compared to pinacyanol cation. The asymmetry is induced by Clv attached to the substituent chain on one side as compared to pinacyanol cation. The asymmetry is induced by Clv and the net non-zero dipole moment of pinacyanol chloride reduces the second ionization potential of pinacyanol. The organic anion like (chloranil)v is attracted by (pinacyanol)2+ dication due to the quaternization of the second nitrogen atom supporting a second CH2CH3 group.
The spectrum of (PC-Cl)-DDQ is also shown (Figure4 (a)).
In this case, there is maximum interaction between pinacyanol chloride and DDQ molecule. The DDQ as compared to chloranil easily forms a semi-quinone ion. The interaction is strongly ionic. There is a very large increase in absorption for hν > Eg. The noise is distributed over a very wide range of absorbed intensity too apart from wide range of frequency or wave number. There is lowering of frequency (wave number) above Eg corresponding to softening of lattice vibrations. There is no shift (compared to blue shift in chloranil complex) in the range of mid-IR spectrum below Eg. This shows absence of anharmonic interaction. The mid-IR envelope is triangular very near to the peak but some kind of imperfect nesting or partial screening dominates for the shape away from the peak. The transition is forbidden direct between 0.25 eV and 0.45 eV with Eg ≈ 0.22 eV but having exponential band tailing below 0.25 eV. There is band tailing between 0.22 eV and 0.25 eV which is
![]() (a)
(a)![]() (b)
(b)![]() (c)
(c)
Figure 4. (a) IR Spectrum of (PC-CL)-DDQ; (b) Nature of transition of (PC-CL)-DDQ; (c) Analysis of gaussian band at low-frequency.
slower function than a step function (Figure 4(b)).
The low frequency Gaussian band around 600 cm−1 is asymmetric showing different slopes of ln(A − AO) vs K-KO on both sides (Figure 4(c)). This Gaussian was symmetric in the chloranil complex. The inverted parabola in transmission again is clearly observed below 1100 cm−1. The forbidden direct transition reveals larger intermolecular distance in DDQ complex.
The spectrum of (PC-Cl)-TCNQ is presented in (Figure 5(a)).
The noise in two regions is observed as usual. There are red shifts of the bands above Eg associated with softening of lattice vibrations. The monotonous featureless absorption above 1700 cm−1 is analysed for absorption function for interband transition. It is found to be the one describing forbidden direct transition with Eg =0.22 eV (Figure 5(b)).
The mid-IR envelope is nearly a triangular distribution near a peak around 1100 cm−1. There is slight increase in transmission and a plateau rather than inverted parabola above 1100 cm−1. Weak Gaussian background absorption is found around 550 cm−1 (Figure 5(c)). The Gaussian band is strongly asymmetric.
The last spectrum is that of (PC-Cl)-TCNE (Figure 6(a)). Apart from usual noise in two regions, the nature of transition is found to be forbidden direct with Eg = 0.225 eV (Figure 6(b)). The low-frequency Gaussian profile is almost symmetric (Figure 6(c)).
The absorption functions are summarized (Table 1).
The low frequency envelopes (Gaussian bands in all cases) are characterized by the full-width at half- maximum governed by strength of electron-phonon interaction and symmetry or asymmetry (Table 2).
The special mid-IR envelopes related with imperfect nesting or partial screening as well as inverted parabola below 1100 cm−1 are also summarized (Table 3).
The materials prepared in the present work has band gap of the order of 0.22 - 0.25 eV. It is known the total number of absorbed photons has maximum saturation value of 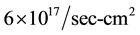 (i.e. per second and per unit area) for Eg ≤ 0.5 eV [26] [27] . For Eg > 0.5 eV, this number drops continuously. Thus the organic semiconductors with Eg < 0.5 eV are very good absorbers of photons. This cannot happen in elemental and compound photoconductors, which has Eg > 0.5 eV. Thus the organic semiconductors can be used as infrared detectors. It is possible to construct other optoelectronic devices such as photodiodes, phototransistors, organic light emitting diodes, etc. using organic semiconductors.
(i.e. per second and per unit area) for Eg ≤ 0.5 eV [26] [27] . For Eg > 0.5 eV, this number drops continuously. Thus the organic semiconductors with Eg < 0.5 eV are very good absorbers of photons. This cannot happen in elemental and compound photoconductors, which has Eg > 0.5 eV. Thus the organic semiconductors can be used as infrared detectors. It is possible to construct other optoelectronic devices such as photodiodes, phototransistors, organic light emitting diodes, etc. using organic semiconductors.
![]() (a)
(a)![]() (b)
(b)![]() (c)
(c)
Figure 5. (a) IR spectrum of (PC-CL)-TCNQ; (b) Nature of transition in (PC-CL)-TCNQ; (c) Analysis of Gaussian at low-frequency.
![]() (a)
(a)![]() (b)
(b)![]() (c)
(c)
Figure 6. (a) IR spectrum of (PC-CL)-TCNE; (b) Nature of transition in (PC-CL)-TNCE; (c) Analysis of Gaussian at low-frequency.
![]()
Table 1. Nature of transition in pinacyanol chloride and its charge transfer complexes.
![]()
Table 2. Low-frequency envelope in pinacyanol chloride and its charge transfer complexes.
![]()
Table 3. Special mid-IR envelope related with imperfect nesting or screening.
4. Conclusions
There are two types of organic photoconductors according to the infrared spectroscopy. In one case, transmission is large and constant above Eg and in the other case, absorption builds up above Eg following direct or indirect transition. In CTCs of Pinacyanol Chloride (PC), the band gap is reduced by 0.03 eV to 0.04 eV, but transition remaining direct type. The mid-IR envelope neither Lorentzian nor Gaussian is triangular type as determined by imperfect nesting and partial screening. Only at low-frequency below 800 cm−1, there is asymmetric Gaussian band in PC which becomes centrally-split Gaussian band in Chloranil, DDQ and TCNE complexes. The central splitting in Gaussian may be arising from density of states or from Davydov-type splitting.
Pinacyanol chloride reveals tunneling of charge carriers through a triangular barrier between 1700 cm−1 and 700 cm−1 arising from imperfect nesting or triangular barrier between valence band and conduction band associated with internal Franz-Keldysh effect (Redfield effect). The material has direct band gap and shows allowed transition. Band gap is somewhat reduced in the four CT complexes. Mid-IR spectrum shows absorption function arising from imperfect nesting or partial screening which is never observed in either binary or ternary CT complexes.
Acknowledgements
Authors acknowledge Department of Physics, Sardar Patel University, Vallabh Vidyanagar, for supporting the work by way of extending laboratory facility, chemicals, instruments and sample characterization charges. Authors are also thankful to management of Gujarat Energy Research & Management Institute (GERMI) for providing necessary permissions to carry out the work at Physics Department and constant encouragement.
NOTES
*Corresponding author.