1. Introduction
Ventral tegmental area dopamine (DA VTA) neurons project their axons to the nucleus accumbens (NAcb) [1] . Increases in the activity of mesoaccumbal dopamine system in the brain are critical for the reinforcing effects of drugs of abuse, such as ethanol and nicotine [2] - [5] . DA VTA neurons have spontaneous rhythmic activity [6] - [10] and the spontaneous activity of midbrain dopamine neurons regulates dopamine release [11] . DA VTA neurons in vivo exhibit burst firing in addition to regular and irregular firing patterns [12] - [14] and burst firing accelerates dopamine release from dopaminergic nerve terminals more effectively than tonic firing [15] [16] . Therefore, the transformation of tonic firing into burst firing in DA VTA neurons likely contributes to reinforcing processes mediatedvia the mesoaccumbal dopamine system.
The properties of burst firing have been studied in midbrain dopamine neurons in vitro (brain slice preparations) as well as in vivo [17] [18] . Both excitatory synaptic activity and intrinsic ionic mechanisms are considered to contribute to the burst firing of DA VTA neurons. DA VTA neurons receive extensive glutamatergic excitatory synaptic afferents from the prefrontal cortex [19] and the burst firing of midbrain dopamine neurons is induced by the activation of N-methyl-D-aspartic acid (NMDA) receptors on these neurons [18] [20] - [23] . The burst firing of midbrain dopamine neurons also depends on two major ion channels: voltage-dependent Ca2+ channels and small conductance Ca2+-activated K+ (SK) channels. Voltage-dependent L-type Ca2+ channels mediate the main depolarizing driving force of spontaneous oscillatory potentials (SOPs), which is around 0.5 Hz, and contribute to the subsequent generation of action potentials (APs) [24] - [26] . A previous study reported that blockade of L-type Ca2+ channels prevents carbachol-induced burst firing in DA VTA neurons [27] . It has been reported that the inhibition of SK current induces burst firing in midbrain dopamine neurons [28] [29] . A previous study reported that concurrent blockade of T-type Ca2+ channels and SK channels by Ni2+ induces burst firing in a subset of midbrain dopamine neurons [30] . Taken together, it is likely that L-type Ca2+ current contributes to the depolarizing driving force that induces burst firing and the SK current counters this L-type Ca2+ channel-mediated depolarizing driving force to prevent burst firing in midbrain dopamine neurons.
Persistent Na+ currents play a critical role in AP generation in neocortical neurons [31] [32] , hippocampal neurons [33] [34] , amygdala neurons [35] , striatal neurons [36] , supraoptic neurons [37] , tuberomammillary neurons [38] , trigeminal neurons [39] , dorsal column neurons [40] and dorsal root ganglion neurons [41] . A recent study has reported that persistent Na+ currents contribute to the initiation of AP generation in midbrain dopamine neurons [42] . It is well known that the potentiation of persistent Na+ currents induces burst firing in neurons, as demonstrated by experimental procedures such as modulation of Na+ channels by phosphorylation [43] - [45] , anemone toxin treatment [46] , or by reduction of extracellular Ca2+ concentrations [47] [48] . Therefore, persistent Na+ currents may generate burst firing in neurons. In midbrain dopamine neurons, Ca2+ current- dependent burst firing has been studied as described above, whereas the properties of Na+ current-dependent burst firing have not been well studied. In the present study, we examined the properties of a persistent Na+ current and whether this current contributes to burst firing in DA VTA neurons.
2. Materials and Methods
2.1. Perspective of the Experiments
All electrophysiological experiments in the current study were conducted using a patch-clamp experimental system in the Department of Physiology and Biophysics, University of Illinois at Chicago from 2005 to 2006.
2.2. Preparation of Dissociated Neurons
Animals used in this study were treated in strict accordance with the American Physiological Society’s Guiding Principles in the Care and Use of Animals and the U.S. National Institutes for Health Guide for the Care and Use of Laboratory Animals. The protocol for all experimental methods was approved by the Institutional Animal Care Committee of the University of Illinois at Chicago. Fisher rats (F-344; 14 - 18 days old; male and female) were decapitated and the brains quickly removed. The brains were placed in an ice-cold cutting solution (in mM: 220 sucrose, 2.5 KCl, 2.4 CaCl2, 1.3 MgSO4, 1.24 NaH2PO4, 26 NaHCO3 and 11D-glucose), which was constantly bubbled with 95% O2 and 5% CO2. Transverse brain slices (400 μm thick) were made on a Vibratome (Series 1000 plus, St. Louis, MO). Brain slices were incubated for 30 min in artificial cerebrospinal fluid (ACSF) (in mM: 126 NaCl, 2.5 KCl, 2.4 CaCl2, 1.3 MgSO4 1.24 NaH2PO4, 26 NaHCO3 and 11 D-glucose; osmolarity 300 mOsm), which was constantly bubbled with 95% O2 and 5% CO2 at room temperature (23˚C - 25˚C). The brain slices were then incubated in a HEPES-buffered external solution (see the next section) containing papain (15 - 18 U/ml) at 32˚C - 36˚C for 20 - 26 min. After papain treatment, the brain slices were further incubated in ACSF for 20 - 40 min. VTA neurons were dissociated with a vibrating stylus apparatus for dispersing cells from the brain slices as previously described [9] . Once the cell dissociation procedure was completed (4 - 7 min), the brain slice was removed from the culture dish, and the dissociated neurons usually settled and adhered to the bottom of the dish within 20 min.
2.3. Nystatin-Perforated Patch Recording
In current-clamp recording, nystatin-perforated patch recording was employed as previously described [8] . Microelectrodes were fabricated on a P-87 puller (Sutter Instrument Company, Novato, CA) from glass capillaries (LE16; Dagan, Minneapolis, MN) and heat-polished on a microforge (Narishige, Tokyo, Japan). The tip resistances of the electrodes were 1.5 - 3 MΩ when filled with pipette solution (in mM: 60 K-acetate, 60 KCl, 1 CaCl2, 2 MgCl2 and 40 HEPES; pH adjusted to 7.2 with KOH, final [K+]i = 131 mM; 290 mOsm). Nystatin was dissolved in methanol at a concentration of 10 mg/ml. This nystatin stock solution was diluted with pipette solution to a final concentration of 100 - 200 μg/ml and the electrodes were backfilled with this solution. A HEPES- buffered extracellular solution contained (in mM) 145 NaCl, 2.5 KCl, 2 CaCl2, 1 MgCl2, 10 HEPES and 11D- glucose. The pH was adjusted to 7.4 with NaOH and the osmolarity was 310 mOsm. The HEPES-buffered solution was constantly bubbled with 100% O2. In some experiments, recording was done in a Ca2+-free external solution, in which Ca2+ was replaced by equimolar Mg2+ to block Ca2+ current. The liquid junction potentials between the pipette and extracellular solutions were estimated to be 5 mV [49] and the results have been corrected by this amount. The osmolarity of the solutions was measured by a Vaprovapor pressure osmometer (Wescor Inc., Logan, UT). Electrophysiological measurements were performed using an Axopatch-1B patch clamp amplifier (Axon Instruments, Union City, CA). Membrane currents and voltage were filtered at 1 kHz and acquired at a sampling frequency of 10 kHz. Data acquisition was performed by a DigiData 1322A interface and pClamp software version 9.0 (Axon Instruments). The dissociated VTA neurons were visualized under phase-contrast on an inverted microscope (Diaphot 300, Nikon, Tokyo, Japan). All experiments were performed at room temperature (23˚C - 25˚C).
2.4. Conventional Whole-Cell Recording
In voltage-clamp recording of persistent Na+ currents, conventional whole-cell recording was employed with the pipette solution (in mM: 60 Cs-methanesulfonate, 80 CsCl, 5 tetraethylammonium (TEA)-Cl, 4 MgATP, 0.3 Na2GTP, 5 EGTA and 20 HEPES; pH adjusted to 7.2 with Tris-OH; 310 mOsm). The extracellular solution to record persistent Na+ current contained (in mM) 100 NaCl, 40 TEA-Cl, 5 KCl, 2 CaCl2, 1 MgCl2, 1 BaCl2, 1 CsCl, 0.2 CdCl2, 0.1 NiCl2, 10 HEPES and 11D-glucose. The pH was adjusted to 7.4 with Tris-OH and the osmolarity was adjusted to 320 mOsm with sucrose. The extracellular solution was constantly bubbled with 100% O2. In some experiments, recording was done in a Ca2+-free external solution, in which Ca2+ was replaced by an equimolar Mg2+. The liquid junction potentials between the pipette and extracellular solutions were estimated to be 8 mV and the results have been corrected by this amount.
2.5. Drug Application
Neurons were continuously bathed in the external solution and drugs were dissolved at their final concentration in the same solution. Drug solutions were applied via a multiple channel manifold (MLF-4; ALA Scientific Instruments, Westbury, NY). Each channel of the manifold was connected to a gravity-fed reservoir with tubing (860 μm, i.d.). The output of the manifold was connected to an outflow tube (500 μm, i.d.), the tip of which was placed within 200 μm of the soma of the recorded neuron. Solution flowed continuously through one manifold channel. Application of drug solutions was controlled by opening or closing valves connected to the reservoirs.
2.6. Source of Drugs and Chemical Agents
The following drugs and chemical agents were used in this study, apamin, BaCl2, CsCl, CdCl2, EGTA, HEPES, nystatin, riluzole, TEA-Cl and tetrodotoxin (TTX) were purchased from Sigma?Aldrich (Saint Louis, MO). Papain was purchased from Worthington (Lakewood, NJ). Riluzole was dissolved in dimethyl sulphoxide (DMSO) and made as a stock solution at a concentration of 100 mM. The stock solution for riluzole was diluted in the HEPES-buffered external solution. The final concentration of DMSO in the HEPES-buffered external solution was 0.01%.
2.7. Data Analysis
Action potentials (APs) were analyzed offline with pClamp 9.0 software (Axon Instruments Inc.). To assess the change of spontaneous firing with drugs, a 60-s-long segment during the peak of the drug response was analyzed as previously described [50] . Inter-spike interval (ISI) histograms were created as previously described [51] . The number of bins was equal to the square root of the number of ISIs. Bin width was obtained by dividing the ISI range (maximum ISI minus minimum ISI) by the number of bins. The coefficient of variation (CV) of the events was obtained by dividing the standard deviation of the event intervals by the mean event intervals. Burst duration was measured between the first spike and the last spike of a spike train. The ISI during burst-like firing was obtained by dividing the burst duration by the number of spike intervals in the burst spike train. The plateau potential, on which the trains of spikes were generated, was measured from the bottom of membrane potential to the threshold of the first spike. The data from cells with AP amplitudes less than 50 mV were discarded. All average values are expressed as mean ± standard error of the mean (S.E.M.). Statistical comparison to assess significant differences was done by the paired or unpaired Student’s t-test. To analyze persistent Na+ current activation, the current was normalized to the maximal current and fitted by the Boltzmann equation, using Origin software version 7 (Origin Lab, Northampton, MA):
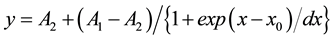
where y is the current at the test voltage of x, A1 is the minimal current and set to be 0, A2 is the maximal current and set to be 1, x0 is the membrane potential for half-activation and dx is the slope factor.
3. Results
3.1. Na+ Current Contribution to Spontaneous Firing of DA VTA Neurons
Figure 1(A) shows typical spontaneous membrane activity before, during, and after the application of 1 μM TTX in DA VTA neurons. Before TTX treatment, the DA VTA neuron exhibited spontaneous firing (Figure 1(A)-a). TTX hyperpolarized the membrane potential and abolished spontaneous firing (Figure 1(A)-b). After the washout of TTX, AP generation reappeared (Figure 1(A)-c). Figure 1(B) shows typical spontaneous membrane activity before, during, and after the application of 10 μM riluzole, a voltage-dependent Na+ channel antagonist, in DA VTA neurons. Before riluzole treatment, the DA VTA neuron exhibited spontaneous firing (Figure 1(B)-a). Riluzole hyperpolarized the membrane potential and abolished spontaneous firing (Figure 1(B)-b). After the washout of riluzole, AP generation reappeared (Figure 1(B)-c).
3.2. TTX-and Riluzole-Sensitive Persistent Na+ Currents
To ascertain the presence of persistent Na+ currents in DA VTA neurons, we used a ramp-voltage protocol with potassium-based pipette solutions and physiological extracellular solutions Figure 2(A1) shows typical currents before and after treatment with 1 μM TTX and TTX-sensitive currents were obtained by the digital subtraction of TTX-insensitive currents from control currents. Figure 2(B1) shows typical currents before and after treatment with 10 μM riluzole and riluzole-sensitive currents were obtained by the digital subtraction of the riluzole-insensitive currents from control currents. The riluzole-sensitive inward current provided a similar current-
![]()
Figure 1. The effects of TTX and riluzole on spontaneous firing in DA VTA neurons. (A1) Spontaneous membrane activity before, during, and after application of 1 μM TTX in a representative DA VTA neuron. No current was injected; (A2) 10-s segments from the longer record shown in A1. Spontaneous activity shown using a faster time scale before (a), during (b), and after treatment with 1 μM TTX (c); (B1) Spontaneous membrane activity before, during, and after application of 10 μM riluzole in a representative DA VTA neuron. No current was injected; (B2) 10-s segments from the longer record shown in B1. Spontaneous activity in a faster time scale before (a), during (b), and after the treatment with 10 μM riluzole (c). A horizontal bar and a dashed line represent the membrane potential of −40 mV.
![]()
Figure 2. Persistent Na+ currents of DA VTA neurons. (A1) Voltage-ramp induced currents before (black line) and after (grey line) treatment with 1 μM TTX in a representative DA VTA neuron. (inset) Slow depolarizing voltage ramp was applied from −90 mV to −10 mV in 2 s (40 mV/s); (A2) TTX-sensitive current obtained by the digital subtraction of TTX-insensitive current from control current; (B1) Voltage-ramp induced currents before (black line) and after (grey line) treatment with 10 μM riluzole in a DA VTA neuron; (B2) Riluzole-sensitive current obtained by the digital subtraction of riluzole-insensitive current from control current. C, Current density- voltage relationship of riluzole-sensitive current in DA VTA neurons (n = 6). Values represent the mean ± SEM.
voltage relationship to the TTX-sensitive current. Figure 2(C) shows the current density-voltage relationship of riluzole-sensitive currents in DA VTA neurons.
3.3. Potentiation of Persistent Na+ Currents with Ca2+-Free Extracellular Solution
We next examined the current-voltage relationship of persistent Na+ current by a voltage-step protocol using cesium-based pipette solution. To identify DA VTA neurons, cell-attached recording was performed before making conventional whole-cell configuration. In the 6 DA VTA neurons examined, average spontaneous firing frequency was 1.5 ± 0.2 Hz. Figure 3(A1) shows currents induced by the voltage-step protocol before and after application of 2 μM TTX in the control extracellular solution. Figure 3(A2) shows currents induced by the voltage-step protocol before and after application of 2 μM TTX in the Ca2+-free extracellular solution. The graph in
![]()
Figure 3. The potentiation of persistent Na+ currents in Ca2+-free extracellular solution. (A1) Currents induced in a representative DA VTA neuron before (top) and after 2 μM TTX treatment (middle) in a normal extracellular solution. TTX-sensitive currents were obtained by the digital subtraction of TTX-insensitive currents from control currents (bottom); (A2) Currents induced in the same DA VTA neuron before (top) and after 2 μM TTX treatment (middle) in Ca2+-free extracellular solution. TTX-sensitive currents obtained by the digital subtraction of TTX- insensitive currents from control currents in a Ca2+-free extracellular solution (bottom). One-second depolarizing voltage steps were applied from a holding potential of −78 mV to −28 mV in 10 mV increments. Persistent Na+ currents were measured at 100 ms before the end of depolarizing voltage steps (open and filled circles). Transient Na+ currents are truncated; (B) Current-voltage relationship of TTX-sensitive persistent Na+ currents in the normal extracellular solution (open circles) and the Ca2+-free extracellular solution (filled circles) (n = 6); (C) Normalized persistent Na+ currents plotted as a function of test voltage before (open circles) and after the elimination of Ca2+ from extracellular solutions (filled circles) (n = 6). Values represent the mean ± SEM.
Figure 3(B) shows the current-voltage relationship of TTX-sensitive persistent Na+ currents in the control extracellular solution and the Ca2+-free extracellular solution. Figure 3(C) shows the normalized persistent Na+ current plotted as a function of test voltage before and after the elimination of Ca2+ from the extracellular solutions. In the control extracellular solution, average half-activating voltage (Vact, 0.5) was −53.6 ± 0.7 mV and average slope factor was 3.4 ± 0.2 (n = 6). In the Ca2+-free extracellular solution, average Vact, 0.5 was −57.1 ± 0.7 mV and average slope factor was 3.7 ± 0.2 (n = 6). The elimination of Ca2+ from the extracellular solution significantly shifted the Vact, 0.5 in a negative direction (P < 0.001) without changing the slope.
3.4. Na+ Current Contribution to Burst-Like Firing
We examined the effect of Ca2+-free extracellular solution, which potentiated persistent Na+ current, on the firing of DA VTA neurons. Figure 4(A1) shows typical membrane activity before, during, and after the application of Ca2+-free extracellular solution with or without continuous hyperpolarizing current injection. In the control extracellular solution, the spontaneous firing frequency was 1.6 Hz (Figure 4(A2), a). The Ca2+-free extracellular solution depolarized the membrane potential and increased the firing frequency to 7.6 Hz (Figure 4(A2), b). When a continuous hyperpolarizing current was injected to counter the depolarizing driving force, burst-like firing was generated in the Ca2+-free extracellular solution (Figure 4(A2), c). When the continuous hyperpolarizing current injection was removed and the Ca2+-free extracellular solution was switched to the control extra-
![]()
Figure 4. Burst firing of DA VTA neurons. (A1) Membrane activity before, during, and after the application of Ca2+-free extracellular solution with or without continuous hyperpolarizing current injection in a representative DA VTA neuron; (A2) 30-s segments from the longer recording shown in A1. Firing patterns in the control (a), in the Ca2+-free extracellular solution (b), in the Ca2+-free extracellular solution with the continuous hyperpolarizing current injection of −41 pA (c) and in recovery (d); (B) Superimposed traces of tonic firing in control (grey line) and burst firing (black line); (C1) ISI histogram for control; (C2) ISI histogram for burst firing. Bin width is 59 ms; (D) Burst firing depends on Na+ current. Burst firing before (top) and after treatment with 10 μM riluzole (bottom) in a representative DA VTA neuron. Horizontal bars and dashed lines represent the membrane potential of −40 mV.
cellular solution, the firing frequency became 1.7 Hz (Figure 4(A2), d). Figure 4(B) shows APs in control and burst-like firing in a faster time scale. In the burst-like firing, a train of spikes rode on a plateau potential and the spike amplitude gradually decreased during the spike train (Figure 4(B)). As shown in Figure 4(C), the firing patterns before and after burst-like firing were analyzed by ISI histograms. The ISI is normally distributed with a peak at 590 ms in control (Figure 4(C1), while the ISI histogram skewed leftward with a wider ISI distribution during the burst-like firing (Figure 4(C2). In 11 neurons out of 16 DA VTA neurons (69%), burst-like firing was generated in the Ca2+-free extracellular solution, when continuous hyperpolarizing current was injected (−48.5 ± 5.6 pA); the average CV of firing frequency was 1.62 ± 0.17. The burst-like firing parameters were as follows: burst duration, 826.5 ± 105.2 ms; spike number in the train, 4.9 ± 0.2; ISI, 221.2 ± 18.9 ms; plateau potential, 10.7 ± 0.6 mV (n = 11). As illustrated in Figure 4(D), the burst-like firing induced by the continuous hyperpolarizing current injection in the Ca2+-free extracellular solution was abolished after treatment with 10 μM riluzole; average membrane potential after treatment with riluzole was −62.3 ± 1.4 mV (n = 4).
3.5. Blockade of T-Type Ca2+ Channels and SK Channels Did Not Induce Burst Firing
In Ca2+-free extracellular solution, reduced Ca2+ influx through voltage-dependent Ca2+ channels decreases Ca2+- activated K+ currents. If this occurred in DA VTA neurons, the counter effect of SK currents would oppose decreases in the depolarizing driving force and burst firing may be induced as previously described in midbrain dopamine neurons [28] [29] . To represent the condition of blocking both voltage-dependent Ca2+ current and SK current in Ca2+-free extracellular solution, we used nickel to produce concurrent blockade of T-type Ca2+ channels and SK channels, since these two ion channels are strongly coupled in midbrain dopamine neurons [30] . We ascertain that blockade of SK channels by 200 n Mapamin reduced AHP in DA VTA neurons (Figure 5(A)). In
![]()
Figure 5. The effects of Ni2+ on the firing of DA VTA neurons. (A) Average 10 of APs before (grey line) and during apamin treatment without current injection (black line). APs are superimposed; (B) Average 10 of APs before (grey line) and during nickel treatment without current injection (black line); (C1) Membrane activity before, during and after the application of 100 μM Ni2+ with or without continuous hyperpolarizing current injection in a representative DA VTA neuron; (C2) 20-s segments from the longer record shown in C1. Firing patterns in the control (a), with 100 μM nickel (b), with 100 μM Ni2+ and the continuous hyperpolarizing current injection of −19 pA (c) and in recovery (d). A horizontal bar and a dashed line represent the membrane potential of −40 mV.
a similar manner, Ni2+ (100 μM) reduced AHP, which is attributable to SK currents (Figure 5(B)). Before nickel treatment, the spontaneous firing frequency was 1.7 Hz (Figure 5(C2), a). The firing frequency was decreased and firing pattern became irregular in the presence of 100 μM Ni2+ (Figure 5(C2), b). When a continuous hyperpolarizing current was injected during nickel treatment, the firing frequency was decreased and abolished without inducing burst firing (Figure 5(C2), c). After the continuous hyperpolarizing current injection was removed and Ni2+ was washed out, the spontaneous firing frequency became 1.6 Hz (Figure 5(C2), d). In 5 DA VTA neurons, continuous hyperpolarizing current injection with nickel treatment failed to generate burst firing; the average continuous hyperpolarizing current was −20.0 ± 3.5 pA.
4. Discussion
In our voltage-clamp analysis, the persistent Na+ current, which was TTX- and riluzole-sensitive, was activated at −60 mV and reached a maximal amplitude at −40 mV in DA VTA neurons. Previous studies have reported that TTX- and riluzole-sensitive persistent Na+ currents are activated at membrane potentials positive to −60 mV in suprachiasmic nucleus neurons [52] , dorsal column nucleus neurons [40] , and neocortex neurons [53] . In our current-clamp analysis, blockade of Na+ current with TTX or riluzole caused membrane hyperpolarization and blocked AP generation in DA VTA neurons. Our findingsindicate that persistent Na+ current is activated at subthreshold membrane potentials and has a critical role in AP generation in DA VTA neurons, supporting the findings of a previous study in midbrain dopamine neurons [42] .
In the present study, the Ca2+-free extracellular solution potentiated persistent Na+ currents by a negative shift of the activation voltage by 3.5 mV without changing the slope factor, indicating that persistent Na+ current in the Ca2+-free extracellular solution can be activated by negative membrane potentials between −63.5 mV and −43.5 mV. It is known that extracellular Ca2+reductions potentiate persistent Na+ currents and this contributes to burst firing [46] [47] . In our current study, burst-like firing induced by the Ca2+-free extracellular solution was completely prevented with 10 μM riluzole, suggesting that the Na+ current is a major depolarizing driving force in these neurons. We found that concurrent blockade of both T-type Ca2+ channels andSK channels by 100 μM Ni2+ did not induce burst-like firing in DA VTA neurons. In the presence of nickel, continuous hyperpolarizing current of −20 pA was able to stop AP generation completely. The burst-like firing of DA VTA neurons in Ca2+- free extracellular solution may be induced by a different mechanism from the reduction of SK currents. This is confirmed by comparing properties of Na+ current-dependent burst-like firing in the current study and those of burst firing induced by the inhibition of SK channels with apamin [28] . The Na+ current-dependent burst-like firing in the present study had short-term spindle-like plateau with small number of APs during the burst, whereas burst firing induced by SK channel inhibition has long-term ramp-like plateau with greater number of APs during the burst [28] .
It is useful to compare the properties of Na+ current-dependent burst-like firing observed in the present study and Ca2+-dependent carbachol-induced burst firing in DA VTA neurons [27] . For Na+ current-dependent burst- like firing parameters, the average burst duration was 827 ms, average spike number per burst was 4.9, and average ISI within the burst was 221 ms. Conversely, L-type Ca2+ current-dependent burst firing induced by carbachol has a longer burst duration (5 - 10 s), a larger number of spikes (more than 10 during the burst) and a longer ISI within the burst (599 ms) [27] . Another difference between Na+ current-dependent and Ca2+-depen- dent carbachol-induced burst firings is a difference in plateau potentials: a spindle-like plateau potential in Na+ current-dependent burst-like firing and a slowly depolarizing plateau-like potential which lasts for 5 - 10 s in Ca2+ current-dependent burst firing [27] . In midbrain dopamine neurons, burst firing induced by the activation of NMDA receptors [22] and by blockade of SK channels with the activation of metabotropic glutamate receptors [29] have similarities with the Ca2+ current-dependent burst firing induced by carbachol [27] . Na+ current-de- pendent burst-like firing in the present study has similarities with findings of a previous study using brain slice preparations, in which average spike number per burst is 3.7 and average ISI within the burst is 73 ms [18] .
A previous study has reported that the concurrent blockade of both T-type Ca2+ currents and SK currents with 100 μM Ni2+ induced burst firing in a subset of midbrain dopamine neurons [30] , whereas 100 μM Ni2+ failed to induce burst firing in the present study. The differences between our findings and those of previous studies may depend on experimental preparations, brain slices, and acutely dissociated neurons. In midbrain dopamine neurons from brain slice preparations, Ca2+ current-mediated SOPs (0.5 - 1.0 Hz), which underlie the generation of spontaneous firing, are prominent and spontaneous Ca2+ spikesare generated by Na+ current blockade in some neuronal populations [24] [25] . In dissociated DA VTA neurons, the amplitude of the SOPs was not prominent (<5 mV) (Figure 1), suggesting that the Ca2+ current contribution to the depolarizing driving force is small in these dissociated neurons. In brain slice preparations, it is likely that Ca2+ currentscontribute to AP generation and Na+ currents supportAP generation. In addition, SK currents counter the Ca2+ current-mediated depolarizing driving force to prevent midbrain dopamine neurons from burst firing in brain slice preparations.
In the present study, the burst-like firing was induced in the dissociated DA VTA neurons under the blockade of Ca2+ channels and the elimination of presynaptic effects on these neurons, however, there is a potential limitation in the experimental design of the study. The burst firing was induced by a non-physyological maneuver such as the elimination of extracellular Ca2+ with hyperpolarizing current injection. Our findings present a possible biophysiological model for burst firing in DA VTA neurons and suggest that both Na+ current- and Ca2+ current-dependent intrinsic ionic mechanisms contribute to burst firing in these neurons. Future work will be necessary to delineate the physiological contribution of these ionic currents to burst firing that increases dopamine transmission more effectively in the mesoaccumbal regions in association with accelerated mediation of reinforcing process.
Acknowledgements
This work was supported by National Institute on Alcohol Abuse and Alcoholism Grant AA05846 (to S.B.A.). This study was partly supported by Grants-in-Aid for Scientific Research (C) (Nos. 22500685 and 25350166 to S.K.) from the Japan Society for the Promotion of Science (JSPS) and the Mishima Kaiun Memorial Foundation, Japan (to S.K.). This study was also supported by funds (No. 106006 to S.K.) from the General Research Institute of Fukuoka University.
Competing Interests
The authors have no competing interests to declare.
NOTES
*Corresponding author.