Phosphorus Adsorption of Some Brazilian Soils in Relations to Selected Soil Properties ()
1. Introduction
Due to the climatic variations impacting the Amazon region of Brazil, the Northeast area of Roraima has transitioned from a semi-arid climatic condition to one with high precipitation [1] . The increase in precipitation has greatly affected the soil’s biogeochemical weathering attributes, erosion and deposition processes, thus contributing to the formation of deep, highly weathered soils dominant in kaolinite and oxidic mineralogy [2] . Some areas of the Amazon have soils originated from mafic rocks (diabase), resulting in the formation of Eurtophic Red Nitosol (Haplustalf), Dystrophic Yellow Red Latosol (Xanthic Haplustox), Eutropic Haplic Tb Cambisols, with A chernozem, Orthic black Chernosol (Mollisol) and vertic Orthic Ebanic Cheronosol (Mollisol) soil series [3] .
Studies have shown that the soils consist typically of kaolinitic mineralogy and low fertility, with the predominant classes being Yellow Latosols (Oxisol) and Yellow Argisols (Ultisol) [4] -[6] . The indigenous areas of Roraima in general are located where these soils are found and reflect certain aspects of the sustainability and quality of life of the indigenes cultivating the soils for agricultural production. In the Raposa Serra do Sol Indigenous Reserve (approximately 1,747,464 ha); the proportion of soils (approximately 19,900 ha) considered as fertile for sustainable agricultural production is situated on gentle undulating relief and includes diabase soils of the “Pedra Preta” Sill [7] .
A major nutritional problem to crops grown in these soils is P deficiency linked to low available P content and capacity to fix fertilizer P in insoluble form. Evaluating the P availability in these soils is important because soil P adsorption capacity directly influences plant response to phosphate fertilization of soils. Phosphorus fixation in soils, widely designated as adsorption, is a slow process that can take years to reach a balance and may lead to decrease in P availability. In most Brazilian soils, studies have shown that the major factors influencing soil P adsorption are the clay fractions, mineralogy, amorphous colloid content, pH, exchangeable aluminum and organic matter [8] -[10] .
Phosphorus adsorption depends on the nature and quality of sites available on the mineral surfaces and is therefore affected by high clay contents within the same mineralogy [11] . Studies by Ker et al. [9] indicate that the mineralogy, crystal size, and specific surfaces of soils will be more important than the quantity of clay in determining soil’s P adsorption capacities [12] -[14] . Bedin et al. [14] state that the presence of large proportions of sesquioxides on clays influences phosphate adsorption and precipitation with iron and aluminum. The objective of this study was to evaluate the maximum P adsorption capacity (MPAC) of the soils developed from mafic rocks (diabase) in northeastern Roraima and its relationships with certain physical, chemical and mineralogical attributes.
2. Materials and Methods
The soils used in this study were sampled from a toposequence and consisted of five soil profiles, developed from mafic rocks that form one of the largest mafic rock bodies in North Amazon’s “Pedra Preta” Sill. The Maloca do Flechal study areas (Figure 1) is inhabited by the Macuxi Indians and situated in the northeast of the state of Roraima, Brazil. The climate average about 1200 mm in rainfall, with sunshine duration of 12 hours/day. The region’s relief is characterized as undulated with step savannah vegetation and patches of seasonal forest on the East-West transect line.
The fieldwork consisted of opening trenches in a toposequence, collecting the soil samples in each profile soil and describing them according to Santos et al. [15] . Soils were classified based on the Brazilian Soil Classification System manual published by Embrapa [16] and analyzed for chemical properties (Table 1) as described in a soil analysis manual published by Embrapa [17] . The profiles were classified as Eutrophic Red Nitosol (Alfisol)- NVe, Tb Eutrophic Haplic Cambisol (Inceptisol)-CXbe; Orthic Ebanic Chernosol (Mollisol)-MEo; vertic Orthic Ebanic Chernosol (Mollisol)-MEov and Distgrophic Yellow Red Latosol (Oxisol)-LVAdf.
The Fe associated with the low crystal minerals in the surface horizons was extracted with ammonium oxalate [18] , and the free Fe extracted using dithionite-citrate bicarbonate (DCB) [19] . The Fe content was determined using induced plasma emission spectrometry while the Fe associated with the crystalline minerals was calculated by the difference between the Fe extracted by DCB (Fed) and the Fe extracted with oxalate (Feo). The mineralogy was determined by X-ray diffraction of the clay and silt fraction. From the natural, Fe-free clay fraction, oriented slides were prepared and irradiated in an X-ray diffractometer at sweeping angle (2θ) intervals between 2˚ and 40˚ and a goniometer speed of 2˚ in 2θ/min, using CuKα radiation with Ni filter [20] . Powdered silt slides were analyzed under the same conditions while the diffraction patterns were interpreted according to Chen [21] .
Phosphorus (P) was evaluated as described by Alvarez and Fonseca [22] using 5 g of fine ground soils mixed with a solution of CaCl2 (0.01 mol∙L−1) containing 50 mg∙L−1 P and shaken for an hour then filtered and the P
![]()
Figure 1. The maloca do flechal study areas.
![]()
Table 1. Chemical characteristics of soils studied.
Hor. = Horizon. 1) pH in water. 2) S = sum of bases. CEC = Cation exchange capacity at pH 7.0. V = percentage of saturation by bases; SOM = Soil organic matter. P = Phosphorus extractable by Mehlich-1. NVe-Red Nitosol. MEo-Orthic Ebanic Chernosol. Cxbe-Tb Eutrophic Haplic Cambisol. MEov-Vertic Orthic Ebanic Chernosol, LVAd-Distrophic Red Yellow Latosol.
concentration using Murphy and Riley [23] procedure. The Langmuir isotherm were obtained by adding 25 mL CaCl2 (10 mol∙L−1) solution containing P in doses ranging from 0 to 60 mg∙L−1. After 24 hours agitation, the samples were centrifuged and the P in the equilibrium solution was quantified colorimetrically [23] .
The data were fitted to the non-linear Langmuir model, relating the concentration of the adsorbed element per adsorption unit (soil) and the concentration of the element in the equilibrium solution (supernatant), and the maximum adsorption capacity calculated. The sorption values of each soil were plotted according to the Langmuir isotherm: C/(x/m) = (1/Kb) + (C/b). Where C, is equilibrium P concentration (mg∙L−1), x/m is the amount of sorbed P (mg∙kg−1), m and b, are the constants related to P sorption maximum (mg∙kg−1) and K is the bonding energy (L∙mg−1), respectively. The plot of C/(x/m) versus C should give a straight line from which 1/b (slope) and K (slope or intercept) can be calculated [24] . The Pearson analysis of simple linear correlation using the Statistic program 7.0 between MPAC and some soil characteristics was carried out, and the correlations greater than 95% were considered significant (α < 0.05).
3. Results and Discussion
The Fe contents determined from successive extractions using DCB (2), ranged from 29.4 (MEov) to 258.7 g∙kg−1 (LVAdf). The values seemed high and atypical of Roraima [5] [6] and Amazonia soils [25] [26] , given that the predominant soils in these environments have iron contents averaging less than 73 g∙kg−1. The LVAdf stood out among the soils studied with lower Feo/Fed ratio indicating the predominance of high crystalline iron oxide.
The soils mineralogical compositions (Table 2) showed the presence of high mineral activities. Illite was common in all the soil horizons but smectite was restricted to soils with the A Chernozem horizon (CXbe and MEov). Kaolinite also occurred generally in most of the profiles, indicating intense weathering with strong lixiviation of bases, low fertility and lower (CEC) especially for LVAdf. The mineralogy of the 2:1 group might have resulted from the geology and climatic variations in the Amazon over geological time. The northeast of Roraima has passed through a recent sub-period of semi-arid climate to the current conditions of greater precipitations [27] [28] . However, this mineralogy contrasts with the data obtained by Vale Júnior [5] and Melo et al. [6] for other Roraima soils.
The P values varied greatly among the soil profiles and horizons. The highest values were observed in the surface horizons (Table 3), ranging from 19.8 (LVAdf) to 50.2 mg∙L−1 (MEo). For the surface horizons, the values ranged from 6.6 mg∙L−1 (LVAdf) to 46.9 (MEov) and this could be attributed to the type of clay mineralogy present in the soils. According to Alvarez et al. [29] , the soils and horizons analyzed presented low P retention, except for LVAdf, which could be attributed to the highly negative charge on the smectites surfaces of the clays. The high P values in most of the soils could be explained by the striking presence of 2:1 minerals. Alves and Lavorenti [30] worked with Latosols in the state of São Paulo, Brazil and reported the lack of correla-
![]()
Table 2. Mineralogical composition of the clay, silt and sand fractions of soils studied.
Qz-Quartz, Ct-Kaolinite, Es-Smectite, Fs-Feldspar, Mi-Mica, Pg-Plagioclase, Af-Amphiboles, Px-Piroxene, Il-Illite, Bt-Biotite, Ac-Actinolite, Cl-Chlorite, Zl-Zeolite, Hb-Hornblendite, Ru-Rutile, Gb-Gibbsite, Gt-Goethite; Hm-Hematite, Mg-Manganite, Cn-Corundum.
![]()
Table 3. Maximum phosphate adsorption capacity for the soils studied.
Nve-Red nitosol, MEo-Orthic ebanic chernosol, Cxbe-Tb eutrophic haplic cambisol, MEov-vertic orthic ebanic chernosol LVAdf-distrophic red latosol.
tions between P and goethite and P and kaolinite. High iron-sesquioxides contents are often considered as the determining factor of P adsorption in soils [31] ; however, the low crystalline forms should fix more P than the crystalline forms [30] . The differences in behavior observed for LVAdf was more related to the presence of gibbsite than to the presence of the low crystalline forms, considering the low Feo/Fed ratio presented by this soil (Table 4).
The MPAC values (Table 3) ranged from 353 to 701 mg∙kg−1 in the surface horizons and from 378 to 1280 mg∙kg−1 for the sub-surface horizons. Except for the values obtained in the MEov profile and the MEo surface layer, the rest of the soils were in the range of MPAC values as indicated by Novais and Smyth [32] for Cerrado Latosols. In Amazon soils, Lima et al. [26] reported MPAC values ranging from 210 to 2170 mg∙kg−1 in Oxisol, while Singh et al. [33] obtained values between 160 and 980 mg∙kg−1 P. For central Amazon, Falcão and Silva [34] reported MPAC ranging from 298 to 888 mg∙kg−1 for Ultisols. The MPAC values were lower than those normally found in highly weathered soils. In basalt-derived soils, Oxisol and Alfisol, Bognola [35] found MPAC values between 1620 and 2740 mg∙kg−1, while Valladares et al. [36] obtained values between 526 and 1.667 mg∙kg−1 for Ultisols. Soils originating from this material have higher MPAC because of the higher clay content and occurrence of oxides such as hematite and goethite [37] [38] . Despite the parental materials, climate and relief contributed to the soil formation with high smectite contents in undulated relief, highly weathered rich Fe and Al oxides contents. The structure, chemical compositions, exchangeable ion type and small crystal size of smectite clays are responsible for the unique properties, including a large chemically active surface area, a high cation exchange capacity and low anion exchange capacity.
In the high clay soils (Table 3), phosphate adsorption was more pronounced. The Yellow Red Latosol (Oxisol) presented the greatest MPAC in the two horizons, which can be explained by the high clay content, high Fed and low Fec contents (Table 4). The Nitosol also presented high MPAC, greater than LVAdf, in the subsurface horizon, which can be due to the high clay content. Vertic Orthic Ebanic Chernosol had the least adsorbed P in the two horizons, which can be explained by the low clay contents and low Fed and Fec values. Generally the soils showed increase in P adsorption with increase in soil depth, which can be attributed to increase in the clay contents from horizon A to horizon B. The high phosphate adsorption in the Latosol and Nitosol in the Amazon soils can be attributed to the low Fe contents predominate in this environment [6] .
The clay, Fed and Fec contents correlated positively and significantly with the MPAC, suggesting that these attributes contributed to an increase in the MPAC in the horizons and profiles studied (Table 5). Given the strict correlations between MPAC and clay, Fed and Fec contents, it can be postulated that the presence of goethite in all the soils was a major determinant for the MPAC and not kaolinite soils. Bahia Filho et al. [39] tested the effects of the mineralogical components on MPAC and observed that goethite (Gt) was mainly responsible, con-
![]()
Table 4. Soil iron and silicon dioxide contents of soils.
Nve-Red nitosol, MEo-Orthic ebanic chernosol, Cxbe-Tb eutrophic haplic cambisol, MEov-vertico orthic ebanic chernosol, LVAdf-distrophic red yellow latosol. Fed-Dithionite-extracted Fe. Feo-Oxalate-extracted iron. Fec-crystalline Fe.
![]()
Table 5. Simple correlations between maximum phosphate adsorption capacity of soils and some soils properties.
T-Clay. Fed-Dithionite-extracted Fe. Feo-Oxalate-extracted iron. Fec-crystalline Fe. n.s.-not significant. *mean of 0.01 < probability < 0.05; **significant at probability < 0.01.
tributing 6% of the total MPAC. The high affinity of geothite soils for P is likely due to the easy access of surface phosphate anions. The 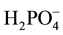 occupies site of the hydroxides (OH−) previously coordinated to Fe3+, forming much more stable surface complexes.
occupies site of the hydroxides (OH−) previously coordinated to Fe3+, forming much more stable surface complexes.
The correlation between the soil organic C and MPAC was not significant for the soils studied. This might be because organic C can influence P adsorption positively in numerous ways [40] , negatively [41] or no effect [42] . The latter has been observed in Cerrado ecosystem (Savanna) soils where the C stock increases linearly with the soil clay and silt contents, demonstrating that total C does not increase the P adsorption, but enhances the richness of the minerals that retain P.
The CEC values and Feo correlated significantly and negatively, respectively, with MPAC. Indeed P adsorption tends to diminish with increase in CEC in the soils, because the negative charges repel the phosphate ion. On the other hand, negative correlation of MPAC with Feo is in line with reported studies. Hernández and Meurer [43] studied three forms of soils in Uruguay, an Argiudoll, a Hapludert, and a Natraqualf, and observed positive correlations between P adsorption and low Fe crystalline forms (Feo).
4. Conclusion
The Oxisol and the Alfisol soils presented the highest phosphate adsorption values, due to Fe and Al oxides rich minerals. The Molissol soils showed the lowest adsorption values due to the high presence of smectite clay. The soils that showed positive correlations with MPAC were in the order: A Horizon-Fec > Fed > clay and B Horizon-clay = Fec > Fed. The soils of the northern border of the Amazon exhibit P adsorption behavior different from other areas of the Amazon. Sustaining agricultural production in these soils will require management approaches that will enhance P availability. An adequate pool of labile P in these soils could be enhanced with inorganic P fertilizations and other organic residues.
NOTES
*Corresponding authors.