Stable isotopes and body composition in children: History, fundamentals, and clinical applications ()
1. INTRODUCTION
The body composition is characterized by the amount of bone tissue, muscle and adipose tissue, also including the organs as well as levels of body water. Your knowledge becomes increasingly important in light of the changes that occur in the nutritional status of various types of diseases and frameworks, such as diabetes mellitus, protein energy malnutrition, in cases of obesity and metabolic syndrome, which seems to be a genuine phenomenon in children, uncommon in non-obese, but highly prevalent among obese children [1].
Increasingly studies related to knowledge of the worldwide phenomenon of childhood obesity are found, such as those conducted by Larsen, et al. (2012) in Danmark [2], Gardner, et al. (2011) in Caribbean [3], Bishwalata (2010) in India [4], Fuiano, et al. (2008) in Italy [5], Amin, et al. (2008) in Saudi Arabia [6], Otero-Gonzáles and García-Fragoso (2008) in Puerto Rico [7], Padula and Salceda (2008) in Argentina [8], Khang and Park (2011) in South Korea [9], Lazarou, et al. (2007) in Cyprus [10] and Abrantes, et al. (2003) in Brazil [11].
The measurement of body composition in children and adolescents is, thus, becoming increasingly common. The normal values for these measures and their relationship with health risk have clinical implications [12]. The current obesity epidemic influences various decisions, with particular focus on body fat percentage (%BF), with urgent establishment of their definitions, rather than relying solely indices involving weight and height. The ability to accurately assess the actual body fat mass (FM) in children is associated with the importance of effective strategies for prevention and treatment of childhood obesity [13].
In vivo techniques do not measure body composition directly, but to predict from measurements of body properties [14]. Thus, the total body water (TBW) can be used and measured by the ingestion of a dose of labeled water [15].
The ideal substance for the determination of the TBW should be a “marker” water which is diffused in all compartments of the body fluid within a short period of time, achieving a uniform and stable equilibrium concentration which can be measured [16,17] . The theoretically ideal marker is the isotope of hydrogen or oxygen, and justifications for the use of deuterium are often presented [17].
The isotopic dilution is particularly useful in children and infants, due to the simplicity of application of the technique and the fact that it could be easily used in field studies. Some disease states may show changes in body hydration and also interfere with the hydration of fat free mass (FFM). These cases should be treated with caution, because they prevent the use of the method as a useful clinical tool [14, 18].
2. METHODS
The subjects were selected scientific articles published in the period between the years 2000 to 2013 indexed in Pubmed database, LILACS, BVS and SciELO, which explored the use of TBW to the knowledge of body composition, especially in children. Furthermore, it was decided to also consider classical studies on the subject, published by the scientific relevance, before the search period.
The main indexing terms used for the search were: body water, deuterium, body composition, doubly labeled water, obesity, children.
This article contains the following sections: “Measurement of body composition”, “TBW and stable isotopes”; “TBW and deuterium”; “TBW and H218O”; “Stable isotopes-Preparation and collection”; “Stable isotopes and laboratory analyzes”, “Results and discussion”, and “Conclusions”.
2.1. Measurement of Body Composition
Models of two-component (2C) are ideal where the intention is to quantify fat and FFM, with greater precision than allowed by simpler methods [14]. Another known model for assessing body composition is the threecomponents (3C) which divides the body into fat, water and remaining dry FFM, which is assumed to have a constant between protein and minerals [19]. The reference method for body composition is the 4-compartment model (4C), which combines the TBW measurements, body density and total body bone mineral density. This is necessary to estimate a fourth component—the %BF, and know the FM and FFM [12].
The model of body composition 4C is more robust to the inter-individual variability in the composition of the FFM, dividing body weight in fat, water, minerals, and protein [20]. However, the criterion 4C is time consuming, difficult to perform and require fasting and is not practical for large-scale projects for young, sick children and is available in just some centers [12].
2.2. TBW and Stable Isotopes
The TBW was evaluated in the nineteenth century, by dissection of cadavers. In the twentieth century methods were introduced using substances/marker compounds such as urea, thio-urea, sulfonamides and antipyrine. However, these methods are not exact because the tracer substances are eliminated in individual rates, thus producing an error in the estimation of individual indefinable body water [21].
Conceptually, one element is defined by the number of protons in their core, but the number of neutrons may differ if different stable combinations of nucleus exist. Most of the chemical elements are a mixture of stable isotopes, which do not disintegrate radioactively. Due to the equal number of protons, these occupy the same isotopes (isos) position (topos) in the periodic table of the elements. However, the stable isotopes of an element is different in its physical, chemical and biochemical characteristics, because of their mass differences, causing thermodynamic and kinetic isotope effects [22].
To assess the body composition of an individual, the body water can be used and measured by the ingestion of a dose of labeled water. The measured isotope enrichment is a function of the amount of body water. The body composition is then calculated from the measured water, based on the coefficient of hydration of the FFM. This method consists in determining the amount of TBW from a known dose of the isotope. Assuming a fixed percentage of body water, and that fat contains no water; it is possible to estimate the FFM. The amount of body fat is the difference between total body mass and FFM [23].
2.3. TBW and Deuterium
In addition to being fully interchangeable with water, diffusible in all compartments of body fluids and to achieve a stable equilibrium uniform, the substance used as a marker for the determination of the TBW should not be selectively stored, metabolized or secreted. There should be no toxic signs or physiological effects caused by marker [16,17].
Among all the isotopes that would be expected to show the greatest differences from the original element, are precisely the hydrogen isotopes. The addition of one or two protons to an atom relatively large is a change insignificant compared to that change experienced by the hydrogen with its nearest isotope—deuterium, because here the extra proton nearly doubles the mass. Indeed, the deuterium is often described as a “new element” [24].
The measurement of TBW through the use of deuterium water was first proposed by von Hevesy and Hofer in 1934 and subsequently applied by Moore 1946 [25], also being developed in the early 1950s. The method, in essence, turns the body into its own recorder metabolic [16]. In the 1930s, large amounts of deuterium had been obtained, and the first studies of its toxicity were performed only a year after its discovery in 1932. Between 1934 and 1939, a total of 216 publications on the biological effects of deuterium appeared [22,26].
When organic substances are synthesized in the presence of heavy water, the atoms of deuterium (D) are incorporated into new molecules in an amount proportional to its concentration in the water body. Since these D atoms are fixed in a stable position, the amount of deuterium incorporated in such organic substances can be measured and, by suitable means, its location in organic molecules can even be determined [16].
However, the volume or space dilution hydrogen, understood as the overall hydrogen switchable [16], is greater than the so-called water space, due to the exchange with the hydrogen of proteins and other constituents of the body. This causes an overestimation of the TBW. The exact error is a debate, but it is generally estimated to be between 1% and 5% [25].
As the deuterium molecule (D2O) has a radius of only 0.1% higher than H2O, the diffusion of the two types of water occurs essentially at the same rate under the same conditions. Thus, it became apparent that heavy water is readily absorbed from the gastrointestinal tract and passes through a variety of animal membranes, virtually the same rate as does the normal water. The easy passage of heavy water using various animal membranes was first demonstrated by Hevesy and Hofer in 1934 in experiments with goldfish [24].
Lucke and Harvey in 1934 also revealed the discovery that the molecule D2O permeates the biological membrane as quickly as water. Edelman, in 1952 showed that 2 hours after application, the concentration of H2O in D2O reached a plateau of identical values in all tissues of the body [21].
Regarding the equilibrium time, the emergence of D2O in blood in the first three hours after oral ingestion establishes graphcally a typical curve, whose level is reached 90 minutes after its ingestion, indicating the stable and homogeneous distribution of D2O in water compartment [21]. Thus, the equilibration time of deuterium oxide with body fluids, both after oral ingestion as subcutaneous injection, is about three hours. The elimination of D2O via the urine, occurs over time, in the form of water [27].
The water molecule is about 75% of body weight, their hydrogen atoms are also easily exchangeable with deuterium in the aqueous phase, and only a fraction of the hydrogen atoms in organic compounds body is interchangeable. From calculations based on these facts, it was observed that hydrogen from water body constitutes at least 95% of the total weight of exchangeable hydrogen. When the total amount of deuterium injected is divided by the concentration in serum after one hour, the resulting figure should represent a precise estimate of TBW. Urinary excretion of deuterium is negligible in the short period of time involved [17].
All the evidence seems to indicate that, when a living organism ingests D2O, there is immediate exchange proportional of D atoms with H of hydroxyl amines, while a very slow connection of D starting with carbons C [24]. Therefore, besides the exchange with water to form deuterium D2O, D atoms are also exchanges with H atoms interchangeable of organic molecules.
Due to the aforementioned changes, which occur rapidly, deuterium injected will be diluted to a volume greater than if were present only in an environment containing water body, which results in an deuterium concentration equilibrium lower than would be observed in the same volume water in vitro [16].
After D2O be present in the body, serum samples may be obtained after a short mixing period and balance. The concentration of D2O is compared with the concentration of D2O injected. By using the basic equation based on the dilution (which relates volume and concentration of the trace before and after the dose) the amount of water in which was mixed D2O is calculated [17]. During the equilibration period, the heavy water must reach the blood cross the capillary membrane, mingle with intracellular and extracellular water, establish trade with free groups H and reach equilibrium with the water stocks, such as cerebrospinal fluid and intestinal contents [16].
In the opinion of Henry Barbour, deuterium provides a “generally unfavorable environment” for biological activity, and exerts “differential effects on the rate of biochemical reactions” [24]. Some of the changes transmitted to the water by deuterium, when hydrogen is replaced to form deuterium oxide, are increases in melting and boiling points, respectively, in 3.82˚C to 1.42˚C. The solubility of sodium chloride is reduced by 15%, the viscosity is increased by about 25%, the steam pressure is reduced and the specific gravity is increased to 1.1074. Some of these properties would certainly be expected to lead to changes in living organisms where substantial proportions of body water are replaced by heavy water [24].
Thus, the toxicity of D2O has been widely investigated. During long-term studies in mice, fertility appears to be impaired if the concentrations of D2O exceed a volume fraction of 0.20, and this effect was completely reversible [28-30] . Significant dips in the growth rate of fibroblasts were found using the volume fractions above 0.20 in vitro [21,31]. Therefore, during the evaluation of shortterm, concentrations of markers D2O should not exceed the volume fraction of 0.01, or 10 g/kg [21]. It was concluded that the threshold for clinically relevant side effects in human adults is between 70 - 140 g of 100% D2O or about 200 - 400 mg deuterium/kg bodyweight. The dosage used in clinical tracer studies is clearly lower and ranges from 1 - 80 mg deuterium/kg bodyweight [22,32]. From 100 g of D2O in humans produces a serum concentration of only 0.2%, an amount used far below the toxic level, and no side effects were observed after administration of D2O to normal individuals or patients [16].
2.4. TBW and H218O
The 18O has been considered an ideal marker for water, because it is not radioactive and toxic [25]. Furthermore, in comparative studies of Lifson and McClintock in 1955 using 2H2O (or D2O) and H218O in mice, H218O was shown to be a more accurate marker for the body water. This probably results from less exchange with nonaqueous constituents of the body [25].
In the 1980s, it was found that the space H218O dilution averaged 3.0% (SD = 0.4) smaller than the space 2H2O dilution, on the basis of subsequent exchanges with non-aqueous hydrogen. For this reason, dilution with H218O should be a more accurate measure of total body water than 2H2O [25].
The time to equilibrate H218O within the body, as evidenced by the threshold values of the respiration and serum, was 1 to 2 hours in normal subjects and from 2 to 3 hours in obese. This equilibration time is similar to that previously observed for D2O [25,33].
In previously reported studies in small animals, rates of distribution of H218O and 2H2O had been very similar [25,34].
The 18O is eliminated from the body as carbon dioxide and water. The difference in the rates of elimination of isotopes, after adjustment to isotopic fractionation, is a measure of the rate of CO2 production, which can be used to calculate the Total Energy Expenditure [27].
2.5. Stable Isotopes-Preparation and Collection
The preparation of dosages and collection of material that will be used is critical for the analysis. Some information is fundamental when it comes to research with stable isotopes, such as security of supply and source of the marker, chemical purity guaranteed and confirmed independently. For intravenous use, solutions must be sterile, pyrogen-free, and should possess stability marking and chemical stability [22].
Analytical errors are determined by the factors that the investigator can manipulate, such as the delivered dose, the duration of the period metabolic, processing of samples and measurement errors during mass spectrometry. Furthermore, the amount of water dose administered to the individual is one of factors which may alter the accuracy of the method. The administration of the very little isotope also can result in a low enrichment of the body fluid at the end of the study, which leads to larger errors in measurement. To ensure delivery of a sufficient dose of isotope, it is typically calculated for each subject, based on individual body weight [27, 35-39] .
Several studies are recognized and use methodologies referenced in relation to the process of dispensing and collecting, verifying several protocols used as Table 1. Are observed uses of 99.8% and 99.9% excess atomic 2H2O [12,40]. By oral administration, dosages are prepared and distributed into individual vials to be ingested by each child. Are also prepared bottles with the individual identification number for collection and storage of saliva samples [23].
Using saliva to measure the TBW, it was determined that its production rate was sufficient to prevent a significant delay in the isotopic distribution compared to plasma [41].
After an overnight fasting, and after emptying the bladder, samples of saliva baseline (pre-dose) are collected [40,42], should be avoided consumption of foods and liquid an hour before and three hours after this moment [21]. The next step is to collect a saliva sample before ingestion of a standard dose of deuterium. Are reported amounts ranging from 2 ml and 3 ml [12,23]. The post-dose samples of saliva may be collected protocols ranging between 1 h and 8 h after ingestion of the dose, according to Table 1. Intervals greater than 3 hours are recommended especially in patients with edema, ascites, pleural effusions, or shock [41].
Some recommendations should also be considered when it comes to preparing and collection, as the permanence of the subjects in the supine position. Another important fact for the accurate detection of concentrations of D2O in the urine or saliva sample is distillation prior to analysis, so as to eliminate all background effects. The absorbance of non-distilled urine is not constant, giving falsely elevated and dispersed. The urine distilled, however, reveals the normal concentration accurately. The absorption of saliva distilled is identical to the absorbance of demineralized water [21].
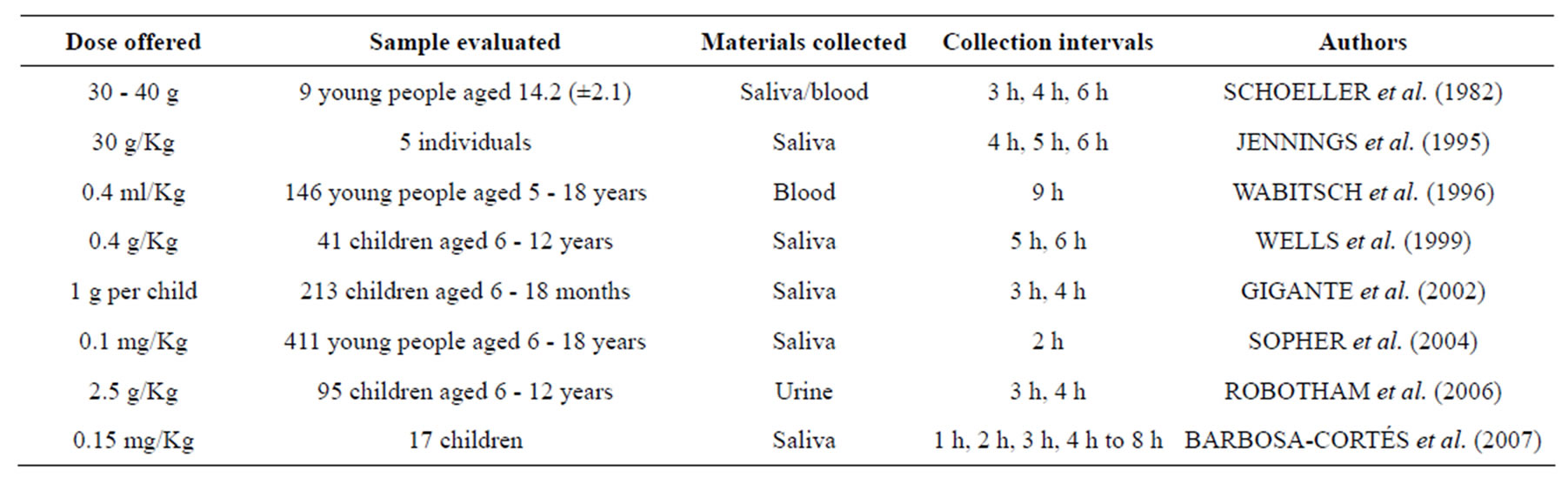
Table 1. Studies on body composition assessment with isotope dilution, considering the dose supplied, samples, materials collected, the collection intervals and authors.
Care must be taken to ensure that the cotton rolls saliva collectors are free of absorbed water. Failure to drycotton can result in a relative error between 1% and 2% to determine TBW because of the dilution of 1 g to 3 g of water absorbed by saliva. Moreover, even though the D2O method ideal for applications in field studies involving either children or infants, attention should be paid also to saliva contamination of the milk, being a potential problem in infants [41].
Periods longer than 3 - 4 h for urine collection are necessary to ensure that the isotopic equilibrium is reached, and the use of serum is less than ideal for pediatric studies because it requires a large volume, besides constituting a set invasive [25].
After collection, there are reports that the samples should be stored at a temperature of approximately −15˚C or −30˚C to blood collected and −70˚C for saliva, and sent for analysis [16,40,42] . The deuterium dilution space is estimated using the plateau method and converted into TBW dividing by 1.04, or more specifically 1.044 [19,40, 43-45] .
2.6. Stable Isotopes and Laboratory Analyzes
The doubly labeled water method is still very sensitive to the precision of isotopic analyzes. The number of participants in a given study by itself does not guarantee that the method is or is not sufficiently developed to correlation analysis, because its accuracy also varies between laboratories [37,38,46,47] .
The measurement of TBW by dilution with deuterium oxide in humans is a well-established method and accurate. Typically, concentrations of D2O can be measured from serum, saliva or urine by infrared spectroscopy, vacuum distillation and infrared absorbance, nuclear magnetic resonance (NMR), gas chromatography and/or mass spectrometry [15,21,22,31] . Using appropriate dosages in humans, enrichments of deuterium are low and must actually be measured by isotope ratio mass spectrometry 2H/H [25].
3. RESULTS AND DISCUSSION
The lean body mass (LBM) is composed of water, minerals and about 73% protein. These data form the basis of most protocols for assessment of body composition, being difficult to measure by simple methods [15].
Another important aspect to consider is that it should be possible to determine changes in the FFM during weight reduction by appropriate programs for this purpose, during which it remains stable, and should preferably be reduced FM [42].
Although there are some considerations about the gold standard for body composition analysis, none of the techniques in vivo can be considered to meet the highest standards of accuracy. In vivo techniques do not quantify body composition directly, but to predict, from measurements of body properties [14].
Apart from the use of TBW for determining the nutritional studies on body composition, it may have clinical applications [25]. Some examples are shown in Table 2. In the case of the group of HIV-infected patients at risk of malnutrition, the assessment of body composition by isotope dilution with deuterium oxide has been shown to be superior when compared to other methods of evaluation [48], including in relation to the bioimpedance-BIA, which underestimated overhydration tissue in adult patients with chronic renal failure [49].
4. CONCLUSIONS
There is a growing consensus that assess body composition in children and adults will raise the profile of health in the countries, an important step to guide interventions in healthcare. The isotope dilution with deuterium oxide is an interesting method because it has low running cost, accuracy, practicality, can be applied under field conditions, poses no risk or discomfort, requires minimal co-

Table 2. Clinical applications of total body water evaluated by isotope dilution with deuterium oxide and associated aspects.
operation from the evaluated, proving to be particularly useful in children and infants, due to the low need for membership. Maybe a useful clinical tool for individuals with normal hydration provides quick results and is safe at recommended doses.
The current references of body composition are based on body mass index assessed in healthy children, but their individual interpretation should be made with caution. The use of stable isotopes, such as deuterium oxide, may constitute a useful tool, safe and more reliable for the determination of body composition. Thus, it would be extremely important to become familiar with this method, which can identify more reliably the overweight and obesity.
5. ACKNOWLEDGEMENTS
We would like to thank CAPES and the Graduate Program in Health Sciences of UFSJ for financial support.