1. Introduction
Streptococcus pneumoniae is a major cause of morbidity and mortality in children, especially in those younger than 5 years of age living in developing countries [1-3]. The main reservoirs of this bacterial pathogen are the nasopharynx (NP) and the oropharynx (OP), from which it can spread directly to adjacent mucosal tissues to cause otitis media (OM) or pneumonia, or through the blood stream to other sites causing sepsis, meningitis or disseminated focal infections. S. pneumoniae is usually isolated in 25% to 60% of NP cultures obtained from healthy children [1-6].
Nasopharyngeal colonization with S. pneumoniae is a pre-requisite for local and systemic disease. Several studies conducted in Israel [6], South Africa [7], Pakistan [8] and Brazil [9] confirm that nasopharyngeal colonization with S. pneumoniae in young children may predict the serotype distribution and antimicrobial susceptibility of the strains producing invasive disease. In a prospective study performed in Costa Rica, 79.2% of children in whom NP cultures were obtained weekly had at least one positive culture for S. pneumoniae during the first year of life [10].
Streptococcus pneumoniae carriage is highest among infants and young children (especially in those <24 months of age) and decreases with age [11]. In the general population in the United States and other industrialized countries, the mean age of S. pneumoniae acquisition is approximately 6 months [12,13], whereas in developing countries, it is as early as 2 to 3 months of age [14,15].
During the past 15 years, a worldwide increase of antimicrobial resistance by S. pneumoniae has been observed, particularly among strains colonizing the respiratory tract or producing respiratory tract infections [16- 18]. Antibiotic-resistance involve mainly few serotypes (6A, 6B, 9V, 14, 19A, 19F and 23F).
Numerous studies have shown that S. pneumoniae conjugate vaccines (PCVs) reduce nasopharyngeal carriage of vaccine serotypes [19-27], however, concerns have been raised because of the increase of certain nonvaccine serotypes such as 19A, 7F and 3 following the introduction of PCV-7 into the NIP of different countries [28,29].
PCV-7 was introduced in Costa Rica in 2004 but its use was limited to high risk patients and the private sector, until 2009 when it was introduced into the NIP (National Immunization Program). Based on the number of PCV-7 doses distributed, it is estimated that less than 5% of the annual birth cohort were fully vaccinated with PCV-7 before NIP introduction.
Our main objectives in this study were 1) to analyze the prevalence and serotype distribution of S. pneumoniae NP and/or OP colonization among Costa Rican children from 6 to 79 months of age, with OM before the introduction of PCV-7 in the NIP of Costa Rica; 2) analyze the antimicrobial susceptibility patterns among the recovered NP and OP S. pneumoniae serotypes isolated; and 3) calculate the potential vaccine coverage against the isolated serotypes with the three currently available PCVs in Latin America (PCV-7, PCV-10 and PCV-13).
2. Materials and Methods
2.1. Study Population
As part of various clinical trials of antimicrobial drug efficacy for OM conducted between 2002 and 2006, a total of 641 Costa Rican children, aged 6 - 79 months, with OM and in whom an NP and/or OP culture were obtained at the time of OM diagnosis, were included in the present analysis.
Participants with at least one of the following ear finding were included in the current analysis: purulent otorrhea of <24 hour duration, at least one otoscopic signs of middle ear effusion: decreased or absent tympanic membrane (TM) mobility; and at least one indication of acute inflammation (ear, pain, marked redness of TM or distinct fullness or bulging TM) and clinical symptoms of OM (fever, tugging or irritability). Participants were excluded from the analysis if the TM was perforated for ≥48 hours; if tympanic tubes were present; and if congenital craniofacial abnormalities or any known immunodeficiency were notified.
All the study protocols were approved by an Institutional Review Board and informed consent was obtained from the parents or legal guardian of each study participant before enrollment.
2.2. Nasopharyngeal and Oropharyngeal Sampling
NP and/or OP samples were obtained in every participant at the baseline visit. Samples were obtained following a deep swab in the NP or OP with a flexible pediatric culture device (Copan Diagnostics Inc, Corona, CA©). Once NP and/or OP samples were collected, they were immediately placed in an Amies medium without charcoal (Copan Diagnostics Inc, Corona, CA©) and transferred to the Research Laboratory for processing. In patients in whom the same S. pneumoniae serotype was isolated from both NP and/or OP, only data from NP was included in the analysis.
2.3. Microbiology
NP and/or OP samples were inoculated onto regular blood agar, blood agar with 5µg of gentamicin, chocolate agar, McConkey agar and manitol salt agar for incubation at 37˚C in 5% CO2 environment for 18 - 72 hours at the bacteriology units of the Centro de Investigaciones Clínicas in Costa Rica. If growth was present, identification was performed by standard procedures. S. pneumoniae identification was performed following the Clinical Committee for Clinical Laboratory Standards by standard procedures [30].
2.4. Antimicrobial Susceptibility
Susceptibility testing for erythromycin and trimethoprimsulfametoxazole (TMP/SMX) was done by Kirby-Bauer disk diffusion. Susceptibility testing for penicillin, ceftriaxone, levofloxacin, amoxicillin, amoxicillin-clavulanate (AM-CL), was determined by minimal inhibitory concentration (MIC) by means of the E-test (PDM Epsilometer, AB Biodisk, Solma, Sweden). Because of the characteristics of the strips and as recommended by the manufacturer, when the E-test method was used, MIC values intermediate between two marks were rounded up to the next higher 2-fold dilution. Interpretation of the results was performed according to the National Committee for Clinical Laboratory Standards recommendations [30].
MIC50 was defined as the MIC of a given antimicrobial drug that inhibited growth of 50% of the isolates, and MIC90 was defined as the MIC of a given antimicrobial drug that inhibited growth of 90% of the isolates. S. pneumoniae isolates with an MIC ≤ 0.06 mg/L were considered susceptible to penicillin; isolates with a MIC value between 0.125 mg/L and 1.0 mg/L were considered intermediate to penicillin and those with a MIC value ≥ 2.0 mg/L were defined as penicillin resistant.
Multidrug resistant (MDR) isolates were defined as those strains resistant to ≥3 different antimicrobial classes.
2.5. Streptococcus pneumoniae Serogrouping and Serotyping
Initially all isolates were stored at −70˚C at Centro de Investigaciones Médicas in Costa Rica in Micro Bank vials (Pro-Laboratory Diagnostics, Austin, TX) and then shipped on dry ice or on transport media at room temperature to the Research Laboratory of the Pediatric Infectious Diseases Unit at Soroka University Medical Center, Beer-Sheva, Israel. Serogrouping and serotyping of all S. pneumoniae isolates were performed in BeerSheva Israel by the Quellung reaction with antisera from Statens Serum Institute, Copenhagen, Denmark [31].
2.6. Statistical Analysis
The statistical package EPI INFO (version 3.5.3) was used to test difference on S. pneumoniae serotyping distribution and microbial susceptibility between children aged ≤ 24 months and >24 months (by the Fisher exact test, Yates or square test, as appropriate). A P value of < 0.05 was considered significant.
Proportion of coverage by PCV-7, PCV-10 and PCV- 13 were calculated by proportion of serotypes included in the vaccines of all serotypes detected in children. No cross protection between serotypes was assumed.
3. Results
Between 2002 and 2006, simultaneous NP and OP samples were obtained from 641 Costa Rican children with OM. The mean age ± standard deviation (SD) was 23 months ± 16.5 months and 393 (61%) children were ≤24 months old. Among the 641 children, a total of 386 S. pneumoniae isolates were detected from 376 (59%) participants (223/376 [59%] ≤ 24 months and 153/376 [41%] > 24 months (P < 0.001)). Among the 386 isolates obtained, 359 (93%) were isolated from NP samples, 22 (6%) were isolated from OP (P < 0.05) and 5 (1%) were isolated in both reservoirs but had a different serotype isolates.
Among the 386 positive NP or OP samples, 229 (59%) samples were obtained among children ≤ 24 months of age (mean age: 13 months) and 157 (41%) samples were from children > than 24 months of age (mean age: 35 months) (P < 0.001).
3.1. Streptococcus pneumoniae Serotypes
Among the 386 S. pneumoniae isolates, 346 (90%) were serotyped. Table 1 shows the distribution of S. pneumoniae serotypes identified. Of the identified serotypes, only serotype 3 was significantly different between the age groups. Overall cumulative PCV-13 serotypes and non-PCV13 serotypes were different between the age groups. As a group, non-PCV13 serotypes were more common in the younger age group than in children >24
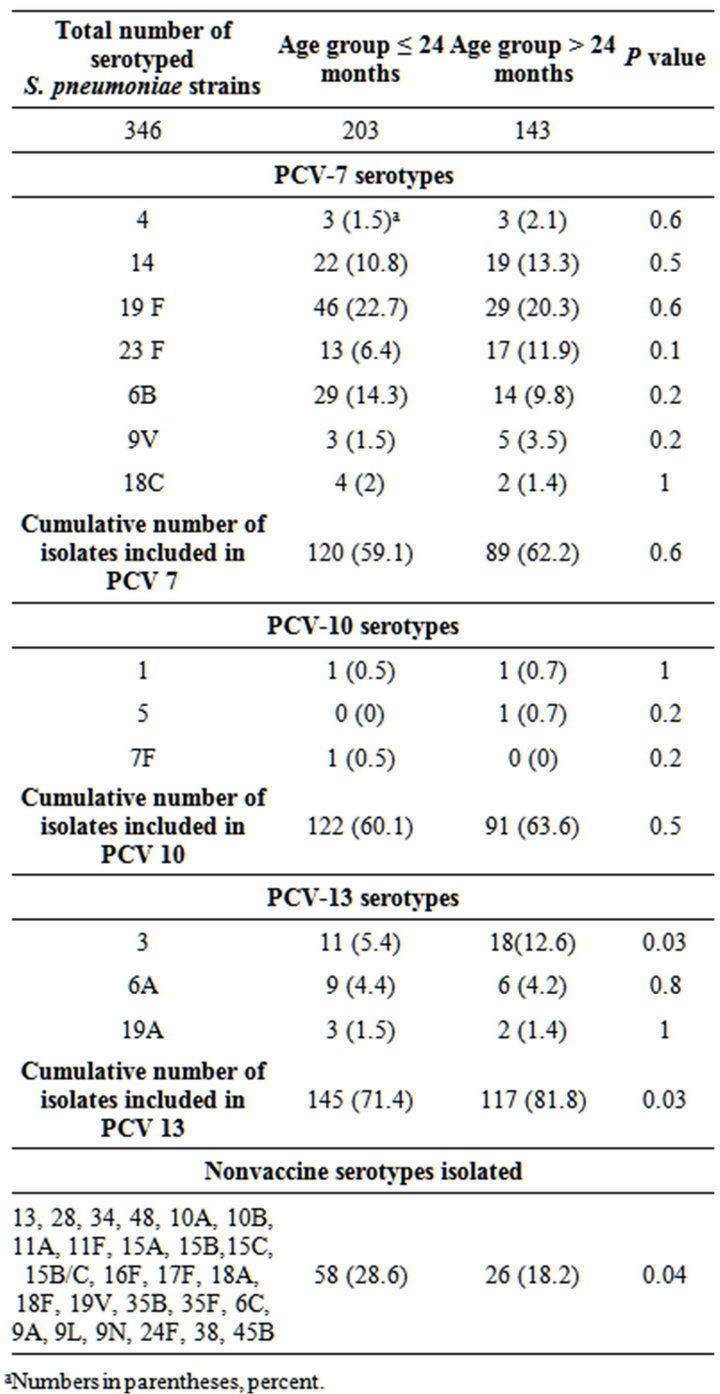
Table 1. S. pneumoniae serotype distribution among isolates obtained from the nasopharynx and/or oropharynx in Costa Rican children with otitis media, between years 2002 and 2006, according to age group.
aNumbers in parentheses, percent.
months old.
3.2 Antimicrobial Susceptibility
Minimal inhibitory concentration (MIC) values for the different antimicrobials tested among S. pneumoniae serotypes included in the three conjugated vaccines are shown in Table 2. Children ≤24 months (203/346 [59%]) had statistically significantly more antimicrobial non-susceptible isolates than children >24 months of age (143/ 346 [41%]) (P < 0.001).
Penicillin non-susceptibility serotypes included in the three conjugated S. pneumoniae vaccines (PCV-7, PCV- 10 and PCV-13) were: 57% in children ≤ 24 months versus a 38% in older children (P < 0.001).
3.3. Multidrug Resistance (MDR)
Among the 346 serotyped isolates, 53 (15%) strains belonging to only 10 serotypes were considered MDR: 4% (2/53) of these MDR strains were non-PCV 13 serotypes and 96% (51/53) were PCV-13 (P < 0.001), Table 3 describes the MDR vaccine-type distribution and their antimicrobial resistance.
3.4. Potential Vaccine Coverage
Potential coverage with the conjugated vaccines, PCV-7, PCV-10 and PCV-13, for the overall study population was: 60%, 62% and 76% respectively, with PCV-13 showing statistical significantly increased vaccine coverage than that observed with PCV-7 (P < 0.001) or PCV-10 (P < 0.001).
Potential vaccine coverage against penicillin non-susceptible isolates was as follows: PCV-7: 59%, PCV-10: 60% and PCV-13: 74% (PCV-7 vs. PCV-10: P: 0.8; PCV-10 vs. PCV-13: P < 0.001; PCV-7 vs. PCV-13: P < 0.001). Potential vaccine coverage against MDR serotypes were as follows: PCV-7: 44/53 (83%), PCV-10: 45/53 (85%) and PCV-13: 51/53 (96%) (PCV-7 vs. PCV-10: P: 0.8; PCV-10 vs. PCV-13: P: 0.04; PCV-7 vs. PCV-13: P: 0.03).
4. Discussion
Streptococcus pneumoniae is the most common cause of vaccine preventable death in children less than 5 years of age globally. In Costa Rica, it is also the most frequent pathogen detected in patients with OM [32].
In the present study, S. pneumoniae colonization of the upper respiratory tract in children with OM was analyzed, 59% of the patients harbor this isolate in the NP or OP. Similar to previous studies, this study demonstrates that the vast majority of colonizing strains were isolated from the NP in comparison to the OP (93% versus 7%, respec-

Table 2. Minimal inhibitory concentration (μg/ml) values among vaccine-type Streptococcus pneumoniae strains isolated from the nasopharynx and or oropharynx of Costa Rican children with otitis media, during 2002-2006.

Table 3. Multidrug resistance among vaccine-type Streptococcus pneumoniae strains isolated from the nasopharynx and/or oropharynx of Costa Rican children with otitis media, during 2002-2006.
tively; P < 0.001), but that 7% of isolates would have been missed if OP cultures were not performed [33-37].
Incidence of colonization, mucosal and invasive disease caused by S. pneumoniae varies by gender, season and age group [36]. In our study and as previously described in other studies [5,6,10,36], children <24 months of age had significantly higher rates of S. pneumoniae colonization than children older than 24 months (59% versus 41%, respectively; P < 0.001). The most common NP and/or OP S. pneumoniae serotypes obtained in this group of Costa Rican children with OM were: 19F, 6B, 14, 23F and 3, comprising 59.6% of the total strains serotyped. The current analysis indicates that in Costa Rica, during the study period, the most frequent colonizing serotype isolated from children at all ages with OM was serotype 19F. These data correlates with previous studies performed in Costa Rican children since 1999 where serotype 19F was also the most common serotype isolated from the middle ear fluid of children with OM [32- 36,38].
The individual serotype distribution was similar among age groups, except for serotype 3, which was significantly more frequent among children >24 months of age than among children ≤ than 24 months of age. Serotypes isolated in children <24 months of age had a statistically significant higher rate of penicillin resistance than those from children >24 months of age. The higher antimicrobial resistance rates in the younger population is similar to previous studies [17-19,21] and also correlates with higher resistance rates observed in S. pneumoniae strains obtained from the middle ear fluid from children ≤ 24 months of age, and supports the need for pneumococcal conjugate vaccination in this age group [6,35,36].
Although serotype 19A was not a frequent pathogen during the study period (5 isolates), it was the most frequent MDR serotype (60%).
Overall potential vaccine protection against the NP and/or OP S. pneumoniae serotypes isolated in this study from Costa Rican children with OM were higher with PCV13 (76%) than that with PCV-10 (62%) or PCV-7 (60%). This difference was mainly driven by the frequency of serotype 3 and the small numbers of serotypes 1, 5, 19A and 7F found. This parallels the serotypes found in OM where serotype 3 is frequent and serotypes 1 and 5 are rarely found as causes of OM, although they can cause invasive disease.
PCV-13 also showed higher serotype coverage rates against penicillin-resistant strains and MDR strains (74% and 96%, respectively) than PCV-10 (60% and 85%, respectively) and PCV-7 (59% and 83% respectively).
To our knowledge, this study represents the largest Latin America set of data analyzing the distribution of S. pneumoniae serotypes obtained from the NP and/or OP of children with OM before the introduction of PCV-7 into the NIP of Costa Rica and following a limited use of PCV-7 into a limited cohort of children. This study serves as a baseline for evaluation of impact of the introduction of PCVs into the Costa Rican NIP.
Currently, three PCVs are in use in Latin America (PCV-7, PCV-10 and PCV-13). Our data analyzing the serotype distribution of S. pneumoniae serotypes colonizing the NP and/or OP of Costa Rican children with OM suggest that, similar to the analysis performed among middle ear fluid isolates in the same population [39], PCV-13 offers the broadest protection against S. pneumoniae strains isolated in this population.
Following the introduction of PCV-13 into the NIP of Costa Rica in 2011, further studies will evaluate the S. pneumoniae serotype dynamics and antimicrobial susceptibility patterns in the NP and/or OP of Costa Rican children.
NOTES