Synthesis and Characterization of New Schiff Bases Formed by Condensation of 2,9-Phenathroline-1,10-dialdehyde with Sulfur-Containing Amines ()
1. Introduction
There is a continuing interest in the design and synthesis of molecules that efficiently bind to DNA and cleave it. In particular, the study of organic chelating agents containing nitrogen and sulfur as the donor atoms and their metal complexes has become a subject of intensive investigation. In this regard, the use of bidentate N,N chelating agents such as 1,10-phenanthroline (phen) has played an important role in synthetic and medicinal chemistry [1]. 1,10-Phenanthroline has also been used extensively as a ligand in both analytical and preparative coordination chemistry as well as in the preparation of many mixed-ligand complexes [2]. 1,10-Phenanthroline and ligands derived from it as well as some of their metal complexes have also been found to be widely used in areas such as molecular catalysis, solar energy conversion, calorimetric analysis, herbicides, molecular recognition, self-assembly, antineoplastic agents, and nucleic acid probes [3]. It has been found that Schiff bases formed by condensation of S-alkyl/aryl esters of dithiocarbazaic acid with heterocyclic aldehydes and ketones contain both “hard” nitrogen and “soft” sulfur donor atoms [4]. Consequently, they are capable of forming stable complexes with a wide vriety of metal ions, some of which have also been found to exhibit interesting physico-chemical properties and potentially useful chemotherapeutic properties [5].
It has also been reported that polymeric metal complexes of 1,10-phenanthroline-based liands containing bithiazole rings exhibit significant magnetic properties [6-10]. Since organic and polymeric magnets offer advantages over traditional magnets because of the diversity of their structures, low density, low magnetic loss and process of preparation without metallurgy at high temperature, studies involving the synthesis of Schiff bases using 1,10-phenanthroline-2,9-dicarboxaldehyde and sulfur-containing primary amines deserve more attention. We, therefore, report here the synthesis and characterization of four new Schiff bases formed from 1,10-phenanthroline-2,9-dialdehyde and some sulfurcontaining amines.
2. General Methods and Procedures
HPLC grade solvents were used in all the reactions. The conventional method (C) of synthesis involves refluxing the reaction mixture for 1 - 3 hours followed by filtration of the solid products using suction filtration.
Conditions for the Microwave method (M) of reaction are as follows: Temp = 75˚C for ethanol and 70˚C for methanol; Reaction Time = 15 minutes, Pressure = 250 (psi), Power = 200 (W).
In all the reactions, 2 - 3 drops of conc. sulfuric acid were used. The solid product that had formed was filtered off using suction filtration. All NMR data were recorded on a 300 MHz Varian NMR Spectrometry. Mass Spectra were obtained on a Varian LC-MS with ESI.
3. Synthesis
We have previously reported the X-ray structure of the solvated 2,9-bis(aminothiophenol)-1,10-phenanthroline that contained two molecules of dichloromethane as lattice solvent [11]. We now report here the synthesis and crystal structure of the dichloromethane-free 2,9-bis (aminothiophenol)-1,10-phenanthroline together with the synthesis of three new Schiff bases formed by condensation of the dialdehyde with amines containing thione or thiol sulfur donor atoms in their backbones. All the compounds have been structurally characterized by spectroscopic as well as X-ray diffraction techniques.
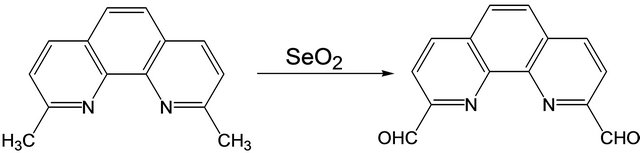
3.1. Synthesis of 1,10-Phenanthroline-2,9- di-carboxaldehyde from 2,9-Dimethyl-1,10- phenanthroline Hemi-Hydrate
1,10-phenanthroline-2,9-dicarboxaldehyde was synthesized from 2,9-dimethyl-1,10-phenanthroline hemi-hydrate following a previously reported procedure [12-14]. The reaction was also carried out under microwave conditions. The yield of the compound was found to be the same as that obtained by the traditional method. However, in the microwave method, the product formed in 10 min compared to 4 hours of refluxing time required in the traditional method. The crude product was recrystallized from hot acetone and dried under vacuum to give the pure compound [Y: 86%] (Scheme 1).
3.2. Synthesis of 2,9-Di-(benzo[d]thiazol-2-yl)- 1,10-phenanthroline from 1,10- Phenanthroline-2,9-dicarboxaldehyde and 2-Mercaptoaniline
2-Mercaptoaniline (4 equivalents) was added to a solution of 1,10-phenanthroline-2,9-di-carboxal-dehyde (1 equivalent) in absolute ethanol (30 mL) containing 2 - 3 drops of conc. sulfuric acid. The solution was refluxed
Scheme 1. Synthesis of 1,10-phenanthroline-2,9-di-carboxaldehyde.
for 3 hr in the conventional method and irradiated for 15 minutes in the microwave method. The reaction mixture was then allowed to cool whereupon the product that had formed was filtered off, washed with ethanol and dried under vacuum. Recrystallization of the crude product from dichloromethane afforded white crystals. Table 1 shows different reaction conditions and percent yields. IR: ν (cm−1) 3100, 3080 (CH aromatic), 1618 (C=C aromatic), 1580 (C=N), 1551 (C=C). 1H-NMR (DMSO-d6): dH = 8.75 (d, J = 8.4 Hz, 8-H, 3-H), 8.70 (d, J = 8.4 Hz, H-4, H-7), 7.30 (d, j = 7.2 H-18, H-18’, 21, 21’), 8.17 (s, H-5, 6), 7.60 (m, H-19, H-19’, H-20, H-20’). 13C-NMR (DMSO-d6, ppm): δC 170, 155, 152, 147, 139, 137, 130, 128, 127, 126, 124, 123, 120. LC-MS (m/z, M+): 451 (M + 5H+), 450 (M + 4H+), 449 (M + 3H+), 447 (M + H+), 446 (M+), 417 (M + 3H+-S), 418 (M+4H+-S), 372 (M + 2H+-C6H4), 359 (M + 3H+-C6H4N), 358 (M + 2H+- C6H4N), 344 (M+-C7H4N), 328 (M + 2H+-C6H4-CS), 314 (M + 2H+-C6H4N-N) (Scheme 2).
3.3. Synthesis of (2,2’)-Dimethyl 2,2’-(1,10- Phenanthroline-2,9-diyl)bis(methan-1-yl-1- ylidene)-bis(hydrazinecarbodithioate) (2) from 1,10-Phenanthroline-2,9- dicarboxaldehyde and S-Methyldithiocarbazate (SMDTC)
SMDTC was prepared following the procedure of Tarafder et al. [15] (Scheme 3).
Table 1. Reaction conditions and percent yields of the schiff base-1.

C—Conventional; M—Microwave.

Scheme 2. 2,9-di-(benzo[d]thiazol-2-yl)-1,10-phenanthroline shiff base.

Scheme 3. Synthesis of (2,2’)-dimethyl 2,2’-(1,10-phenanthroline-2,9-diyl)bis(methan-1-yl-1-ylidene)-bis(hydrazinecarbodithioate) schiff base.
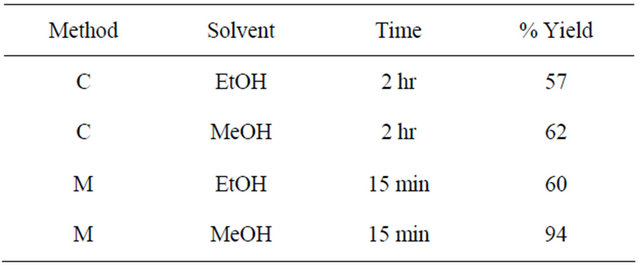
SMDTC (0.12 g, 0.99 mmol) was added to a solution of 1,10-phenanthroline-2,9-dicarboxaldehyde (0.09 g, 0.33 mmol) in 30 mL absolute ethanol containing 2 drops of conc. sulfuric acid. The solution was refluxed for 2 hr and then allowed to cool to room temperature. The solid product that had formed was filtered off, washed with ethanol and dried under vacuum. The crude product was recrystallized from dimethylsulfoxide to obtain white crystals suitable for X-ray diffraction. Yields are shown in Table 2 for various reaction conditions and solvents. The highest yields were obtained using methanol as the solvent: IR: ν (cm−1). 3200 (NH), 3200 (N-H), 3100, 2900 (CH aromatic and aliphatic), 1580 (C=N), 1500, 1600 (C=C, aromatic), 1050 (C=S). 1H-NMR (DMSO-d6, ppm): δH 13.71 (s, 2 NH), 8.28 (d, J = 8.7 Hz, 2H), 8.58 (d, J = 8.7 Hz, 2H), 8.60 (s, 2H), 8.07 (s, 2H), 2.01 (s, 6H). 13C-NMR (DMSO-d6, ppm): δC 206, 199, 146, 139, 129, 127, 126, 119, 30. LC-MS (m/z, M+): 446 (M + 2H+), 445 (M + H+), 444 (M+), 397 (M + H+-CH3SH), 386 (M-C2H2S), 346 (M + H+-2CH3SH), 266 (M + 2H+- 2C2H2S2) (Figure 1).
The results in Table 2 above indicate a significant increase in % yield in the microwave method using methanol as a solvent.
3.4. Synthesis of (2,2’)-Benzyl 2,2’-(1,10- phenanthroline-2,9-diyl)bis(methan-1-yl-1- ylidene)-bis(hydrazinecarbodithioate) (3) from 1,10-Phenanthroline-2,9- dicarboxaldehyde and S-Benzyldithiocarbazate (SBDTC)
SBDTC was prepared using a procedure described by Audrieth et al. in a similar synthesis [16]. SBDTC (0.198 g, 1.00 mmol) was added to a solution of 1,10-phenanthroline-2,9-dicarboxaldehyde (0.12 g, 0.50 mmol) in 30 mL absolute ethanol followed by the addition of two drops of conc. sulfuric acid. The reaction mixture was refluxed for 3 h, then left to cool to room temperature. The product that had formed was filtered off, washed with ethanol and dried in vacuum. White crystals of the compound were obtained by recrystallizing the crude product from dimethylsulfoxide. Yields in ethanol and methanol under different reaction conditions are given in the Table 3. Diffraction quality crystals for X-Ray structure determination were obtained by recrystallizing the compound from dimethylsulfoxide. IR: ν (cm−1): 3200 (N-H), 3029 (=CH aromatic), 2900 (C-H aliphatic), 1572 (C=N), 1500, 1605 (C=C), 1056 (C=S). 1H-NMR (DMSO-d6, ppm): δH 13.80 (s, 2NH), 8.63 (s, 2H), 8.56 (d, J = 8.5 Hz, 2H), 8.27 (d, J = 8.5 Hz, 2H), 8.08 (s, 2H), 7.48 (d, J = 7.1 Hz, 4H), 7.38 (dd, J = 7.1 Hz & 7.2 Hz, 4H), 7.33 (m, 2H), 4.6 (s, 2H). 13C-NMR (DMSO-d6, ppm): δC 197, 163, 153, 147, 145, 137, 136, 129, 127, 119, 56. LC-MS (m/z): 599 (M + 3H+), 598 (M + 2H+), 597 (M + H+), 596 (M+), 474 (M + 2H+-PhCH2SH), 473 (M + H+-PhCH2SH), 349 (M + H+-2CH3SH), 399 (M + 3H+-C13H12S), 350 (M + 2H+-2PhCH2SH), 321 (M + H+- 2CH3SH-N2), 293 (M + H+-2CH3SH-2N2) (Scheme 4 and Figure 2).
Table 2. Reaction conditions and percent yields for the synthesis of schiff base-2.

Table 3. Reaction conditions and percent yields for the synthesis of schiff base-

Scheme 4. Synthesis of (2,2’)-benzyl 2,2’-(1,10-phenanthroline-2,9-diyl)bis(methan-1-yl-1-ylidene)-bis(hydrazinecarbodithioate) schiff base.

Figure 1. ORTEP diagram of (2,2’)-dimethyl 2,2’-(1,10-phenanthroline-2,9-diyl)bis(methan-1-yl-1-ylidene)-bis(hydrazinecarbodithioate) (2). Solvent molecules not included for clarity.

Figure 2. ORTEP diagram of (2,2’)-benzyl 2,2’-(1,10-phenanthroline-2,9-diyl)bis(methan-1-yl-1-ylidene)-bis(hydrazinecarbodithioate) (3). Solvent molecules not included for clarity.
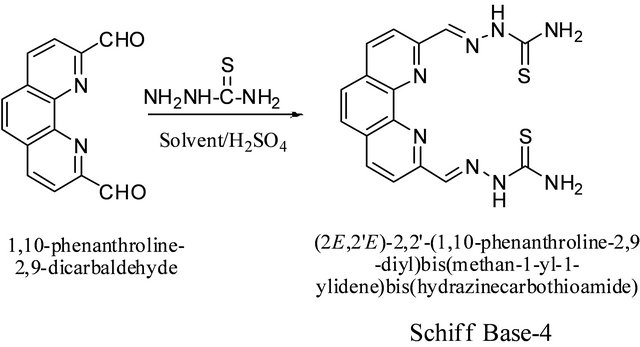
3.5. Synthesis of (2,2’)-2,2’-(1,10-Phenanthroline- 2,9-diyl)bis(methan-1-yl-1-ylidene)bis- (hydrazinecarbodithioate (4)) from 1,10-Phenanthroline-2,9-dicarboxaldehyde and Thiosemicarbazate Scheme 5
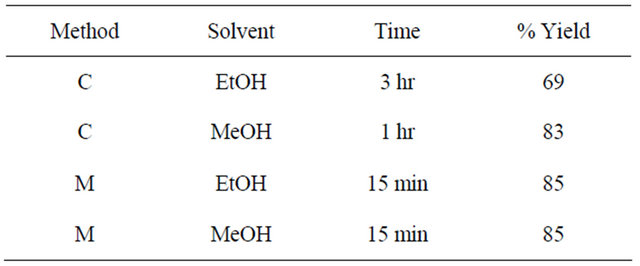
The compound was prepared using the same procedure as used for 4. Yields in ethanol and methanol under different reaction conditions are given in the Table 4. Thiosemicarbazide (0.136 g, 1.5 mmol) was used instead of S-benzyldithiocazate. IR: ν (cm−1): 3300, 3250 (NH2), 3153 (=CH), 1580 (C=N), 1046 (C=S). 1H-NMR (DMSOd6, ppm): δH 12.02 (s, 2NH), 8.78 (d, J = 8.2 Hz, 2H), 8.60 (d, J = 8.2 Hz, 2H), 8.50 (s, 2H), 8.08 (s, 2H). 13C-NMR (DMSO-d6, ppm): δC 178, 154, 151, 145, 140, 137, 128, 120. LC-MS (m/z): 384 (M + 2H+), 383 (M + H+), 382 (M+), 366 (M + H+-NH3), 354 (M + 2H+-N2H2), 349 (M + H+-2NH3).
Methanol was found to be a better solvent in the synthesis of the Schiff base in which thiosemicarbazide was used as one of the reactants. Both microwave and conventional methods produced the same % yield. However, microwave conditions used only 15 minutes.
X-ray structures of the Schiff bases show that the azomethine nitrogen atoms in the molecules are trans to
Scheme 5. Synthesis of (2,2’)-2,2’-(1,10-phenanthroline-2,9- diyl)bis(methan-1-yl-1-ylidene)bis-(hydrazinecarbodithioate) schiff base.
Table 4. Reaction conditions and percent yields for the synthesis of schiff base-4.
the phenanthroline ring nitrogen atoms but the thione sulfur atoms are cis to them. In this configuration, the Schiff bases cannot act as hexadentate chelating agents using the two phenanthroline ring nitrogen atoms, the two azomethine nitrogen atoms and the two thione sulfur atoms. However, in solution, rotations about the C-C and N-N bonds of the hydrazinic side chains are possible and in this way, all the six donor atoms can take up positions suitable for coordination with a metal ion.
4. X-Ray Structure
Crystals of the Schiff bases 2 and 3 were grown from DMSO. Crystals of dimensions 0.36 × 0.21 × 0.12 mm3 and 0.22 × 0.11 × 0.03 mm3 for 2 and 3, respectively were mounted on a MiTeGen loop with Apiezon grease at ambient temperature. Diffraction study was done on a Rigaku XtaLab mini bench-top instrument. Data collection and data reduction was done with the software of the instrument “Crystal Clear” [17]. The radiation source was MoKα (λ = 0.71075 Å). Schiff base 3 crystallized out of solution as platelets as can be seen from the crystal dimension, which had affect on data quality. Both the compounds were crystallized in a triclinic crystal system with P-1 space group. Unit cell dimensions (Schiff base 2): a = 9.826 (3) Å, b = 11.581 (3) Å, c = 14.034 (4) Å, α = 107.671 (8)˚, β = 100.278 (7)˚, γ = 99.610 (7)˚ and Volume 1455.0 (7) Å3. Unit cell dimensions (Schiff base 3): a = 11.841 (5) Å, b = 12.204 (5) Å, c = 15.361 (6) Å, α = 80.986 (6)˚, β = 70.997 (5)˚, γ = 88.934 (6)˚ and Volume 2071.6 (15) Å3.
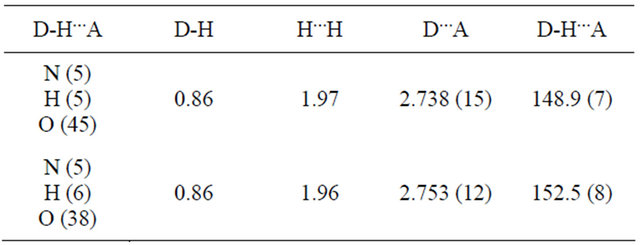
Structure Solution and Refinement: The structure was solved by direct methods [18] and expanded using Fourier techniques. The non-hydrogen atoms were refined anisotropically. Hydrogen atoms were refined using the riding model. The final cycle of full-matrix least-squares refinement on F2 was based on 3117 and 4187 observed reflections, 325 and 469 variable parameters for Schiff bases 2 and 3 respectively. Which converged with unweighted and weighted agreement factors of: R1 = 0.0358 and wR2 = 0.0839 for Schiff base 2 and R1 = 0.0988, wR2 = 0.2353 for Schiff base 3. This was conducted using the program suite WINGX [18]. The compounds crystallized as solvates. Schiff base 2 had two DMSO molecules and Schiff base 3 had three DMSO solvent molecules per asymmetric unit. One of the DMSO molecules in Schiff base 2 was disordered with the sulfur atom occupying two sites each as the inversion of the other with site occupancy factors of 0.7 and 0.3. The other DMSO molecule is located within the range of hydrogen bonding interaction with the H5 atom on N5 atom of the molecule for Schiff base 2. For Schiff base 3, two of the DMSO molecules are within the range of hydrogen bonding interaction with the hydrogen atoms on N5 and N6 atoms of the molecule.
Schiff base 2.

Schiff base 3.
5. Conclusion
Four new Schiff bases of 2,9-dimethyl-1,10-phenanthroline hemi-hydrate with sulfur-containing amines have been successfully synthesized. Significant reduction in reaction time has been observed when the CEM microwave technology was used. In some cases, methanol was found to be a better solvent than ethanol. However, reaction of the dialdehyde with 2-mercaptoaniline in MeOH did not lead to the formation of the cyclic product in high yield. Due to the higher polarity of MeOH, the oxidative elimination of H2 was not favorable.
6. Acknowledgements
We thank the Department of Chemistry at Tennessee State University for providing the necessary support to carry out the research. We also thank the Department of Education, Title III funds for providing instrumental support.
NOTES