Antimicrobial Activity of Eucosterol Oligosaccharides Isolated from Bulb of Squill (Scilla scilloides) ()
1. Introduction
Scilla is a genus of roughly 50 to 80 bulb-forming perennial herbs which consist of about 81 species. The genus has been currently classified as belonging to family Asparagaceae, subfamily Scilloideae which was formerly treated as a separate family, Hyacinthaceae [1]. The plant is known to be native to woodlands, subalpine meadows, and seashores throughout Europe and Asia [2]. Squill [Scilla scilloides (Lindl.) Druce, syn. Barnardia scilloides] has been used as a traditional Chinese medicinal plant showing antidote effect, blood circulatory activation, cough control and abscess reduction [3]. Especially, the root extract has been known to have anti-inflammatory and antioxidative effects [4]. Scillascillosides, eucosterol oligoglycosides (EOs) isolated from the squill plant, were found to exhibit cytotoxicity against several tumor cells [5]. Especially, EOs isolated from S. scilloides have been proved to have inhibitory effect on 12- O-tetradecanoylphobol-13-acetate (TPA) stimulated 32P incorporation into phospholipids of HeLa cells [6,7]. Scillasaponins A, B and C as triterpenoid oligosaccharides, and lanosterol oligosaccharides were isolated from the plants of the subfamily Scilloideae including S. peruviana [8,9]. Recently, anti-tumor activity of EOs isolated from S. scilloides has been also reported [10].
Pesticides may adversely affect humankind, are harmful to the environment, and make disrupt the natural equilibrium among microbial communities. Because natural antagonistic products derived from plants are commonly environmentally sound in disease control and food preservation, their use is now being recognized worldwide as an alternative for sustainable agriculture.
In the course of our screening for various bioactivities of native plant resources in Korea, we isolated four eucosterol oligosaccharides from bulb of S. scilloides. Herein we present antimicrobial activity of four eucosterol oligosaccharides including E-1, E-2, E-3 and G-1 compounds against bacteria, fungi and alga.
2. Materials and Methods
2.1. Purification and Analysis of Active Compounds
The fresh bulbs of S. scilloides (2.5 kg) were extracted with MeOH at room temperature (7 days ´ 3) to give an extract (27 g) as shown in Figure 1. The MeOH extract was suspended in H2O (1 L) and then shaken with EtOAc (1 L ´ 2, each time), n-BuOH saturated with H2O (1 L ´ 3, each time), successively. The n-BuOH (10 g) fraction was again divided into twenty fractions (Fr. 1 - Fr. 20, each 100 ml) by gel filtration on Sephadex LH 20 (3.0 ´ 70 cm, 210 g) eluting with MeOH. The fractions 3-5 (6 g)

Figure 1. Purification procedure for eucosterol oligosaccharides (EOs) including Scillascilloside compounds E-1, E-2, E-3 and G-1 from Scilla scilloides.
were preparative-HPLC on YMC-Pak ODS-AQ column (300 ´ 10 mm I.D. 120A) eluting with aqeous MeOH (75%, 1.5 ml/min, det. at 210 nm) to give fifteen sub-fractions (Subfr. 1 - Subfr. 15, each 50 ml). These sub-fractions were further purified on preparative-HPLC eluting with 75% aqeous MeOH to obtain compounds E-1 to G-1 (100 mg, from Subfr. 5’ to 6’), E-2 (18 mg, from Subfr. 8’) and E-3 (12 mg, from Subfr. 10’ to 11’). Melting points were determined on Electrothermal Melting Point Apparatus. Optical rotations were measured on DIP-370 Digital Polarimeter (JASCO). IR spectra were measured on IR Report-100 infrared spectrophotometer (JASCO). HRFABMS spectra were measured on JMS 700 Mass (JEOL) and ESI Mass spectra were obtained on a VG Quattro 400 Mass (FISONS). 1Hand 13C-NMR spectra were recorded on a AC 300 MHz and DMS 600 MHz (BRUKER) the chemical shifts being represented as part per million (ppm) referenced to solvent signal. Column chromatography was carried out using Kieselgel 60, 400 - 230 mesh, (MERCK). TLC was performed on aluminium backed kieselgel 60 GF254 plates (Merck) developed with BuOH/MeOH/H2O (vol, 4:1:1), and spots were visualized under UV light and by 10% sulfulic acid (in H2O) followed by heating. The compounds were identified as scillascilloside E-1, E-2, E-3 and G-1, which had previously been isolated from this plant, on the basis of their spectral and physical data in comparison with those reported in literature [6,10]. Sciscllascilloside E-1: white amorphous powder (aq. MeOH), mp 221˚C - 223˚C, [a]25 D: −57.1˚ (c 0.08, MeOH). ESIMS (m/z): 1245.6 (M + Na)+, 1221.7 (M − H)-, MW. 1222. Scillascilloside E-2: white amorphous powder (aq. MeOH), mp 210˚C - 216˚C, [a]25 D: −38.1˚ (c 0.08, MeOH), ESI-MS (m/z): 1247.7 (M + Na)+, 1223.7 (M − H)-, MW. 1224. Scillascilloside E-3: white amorphous powder (aq. MeOH), mp 218˚C - 221˚C, [a]25 D: −51.9˚ (c 0.08, MeOH), ESI-MS (m/z): 1303.6 (M + Na)+, 1219.6 (M − H)-. MW. 1220. Scillascilloside G-1: white amorphous powder (aq. MeOH), mp 240˚C - 245˚C, [a]25 D: −46.8˚ (c 0.08, MeOH), ESI-MS (m/z): 1570.2 (M + Na)+, 1546.2 (M − H)-, MW. 1547 (Figure 2).
2.2. Antimicrobial Activity Test
The antimicrobial activity of the crude methanol extracts from the bulb of S. scilloides and the purified materials, eucosterol oligosaccharides, against bacteria, fungi and alga was evaluated by the paper disc method. All samples were dissolved in trace ethanol. Sterile filter paper discs (Whatman No. 1, 8 mm diameter) were impregnated with 250 µg and 500 µg of each sample (50 µl) per paper disc and dried under the laminar flow cabinet overnight. Seeded agar plates were prepared and inoculated with 0.1 ml of each inoculum, and the paper discs were placed on the plates. The test microorganisms used in this study were Bacillus subtilis KCTC 1914 (Korean Culture Type Collection, KRIBB, Daejeon), Escherichia coli KCTC 1924, Salmonella typhimurium KCTC 1926, Staphylococcus aureus KCTC 1916, Staphylococcus aureus KCTC 1928 (as a MRSA bacterium), Streptococcus mutans DSM 6178 (Deutsche Sammlung von Mikroorganismen, Braunschweig, Germany), Aspergillus flavus EML-AF01, Pyricularia oryzae EML-PO1, Chlorella regularis EML-CR02 (Environmental Microbiology Lab, Chonnam National University, Gwangju, Korea) and Candida albicans KCTC 1940 (Korean Collection for Type Culture, KRIBB, Daejeon, Korea). The medium for B. subtilis and S. aureus was Nutrient agar (pH 7.0) containing (g/l) peptone, 5; meat extract, 3; agar, 15. The medium for C. albicans was YMPG agar containing (g/l) yeast extract, 3; malt extract, 3; soybean peptone, 5; glucose, 10; agar, 15. The medium for A. flavus was YpSs agar containing (g/l) yeast extract, 4; soluble starch, 15; K2HPO4, 1; MgSO4∙7H2O, 0.5; agar, 15. The medium for C. regularis was Arnon’s A5 medium (pH 6.5) consisting of 1 ml Arnon’s A5 solution containing (g/l) KH2PO4, 1; MgSO4∙7H2O, 1; FeSO4∙7H2O, 0.005; yeast extract, 5; glucose, 20; agar, 20. All assays were performed in duplicate, so that four inhibition zone measurements were obtained for each test combination. These values were averaged to obtain the final inhibitory activity results. For each assay, two control plates were inoculated with ethanol, but without actual extracts, and were treated in the same manner as the test plates.
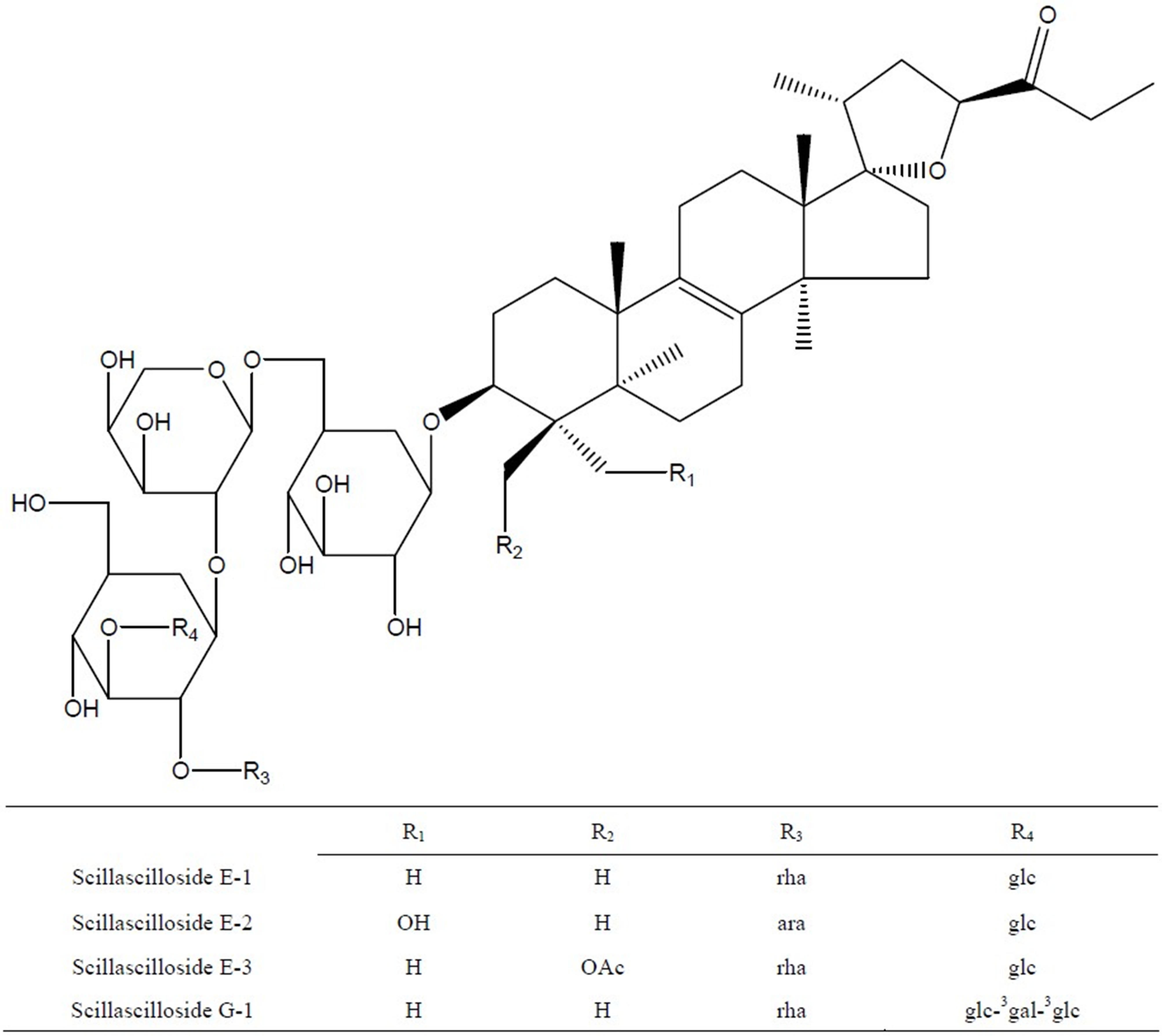
rha: α-L-rhamnopynosyl, ara: α-L-arabinofuranosyl, glc: β-D-glucopyranosyl, gal: β-D-galactopyranosyl.
Figure 2. Chemical structure of eucosterol oligosaccharides (EOs) including Scillascilloside E-1, E-2, E-3 and G-1.
3. Results and Discussion
The use of and search for anticeptic drugs and preservatives derived from plants have accelerated in recent years [11-13]. This study evaluated the antimicrobial activities of the methanol extract from the bulb of S. scilloides plant as well as the purified eucosterol oligosaccharides against bacteria, fungi and alga. The methanol extract from the bulb of S. scilloides was also highly active against fungi but little against bacteria tested (Table 1). As shown in Table 2, the purified eucosterol oligosaccharides including E-1, E-2, E-3 and G-1 from the bulbs were selectively active against eukaryotic cells including three fungal species such as A. flavus EML-AF01, C. albicans KCTC 1940, P. oryzae EML-PO1 and an alga such as C. regularis EML-CR02 at the concentration of 200 µg per paper disc, producing an inhibition zone of up to 26 mm, but hardly active against bacteria including B. subtilis KCTC 1914, two strains of S. aureus KCTC 1916 and 1928, and S. mutans DSM 6178. Out of four purified EO compounds, Scillascilloside E-3 revealed the highest inhibitory activity against fungi and alga (Table 2).
In a previous research related to synthesis of antitumor saponins, eucosterol glycoside which is a lanosterol oligosaccharide, isolated from pineapple lily (Eucomis bicolor) demonstrated antitumor activity by causing 44% inhibition of TPA-stimulated 32P incorporation into phospholipids of HeLa [6,13]. Generally, the synthesis of structurally complex natural products provides not only stringent tests of known methods and reactions but also opportunities to devise new synthetic methods and strategies. Aoyama and his coworkers of Nagoya City University have synthesized the oligosaccharide subunit of scillascilloside E-1, focusing on the synthesis of its aglycon. Demands for plant-derived chemicals have been increasing in the broad industry fields including not only botanical extracts used in herbal supplements and pharmaceutical sources but also natural antifungal agents in environmentally friendly agriculture.
Our results indicated that the methanol extract as well as the purified eucosterol oligosaccharides from the medicinal plant, S. scilloides, can be applied as a natural fungicidal agent or a food preservative for control of molds. More studies on the mode of action, structure activity of the EO derivatives, and their activity spectra against various eukaryotic cells are needed in the future.
Table 1. Inhibitory activity of methanol extract from bulb of Scilla scilloides against microbes.

*The inhibitory activity was evaluated at 500 µg of MeOH extract per paper disc (8 mm). +: slight inhibitory activity. −: no inhibitory activity. ++: moderate inhibitory activity. +++: high inhibitory activity.
Table 2. Inhibitory spectrum of EOs purified from bulb of Scilla scilloides against bacteria, fungi and alga.
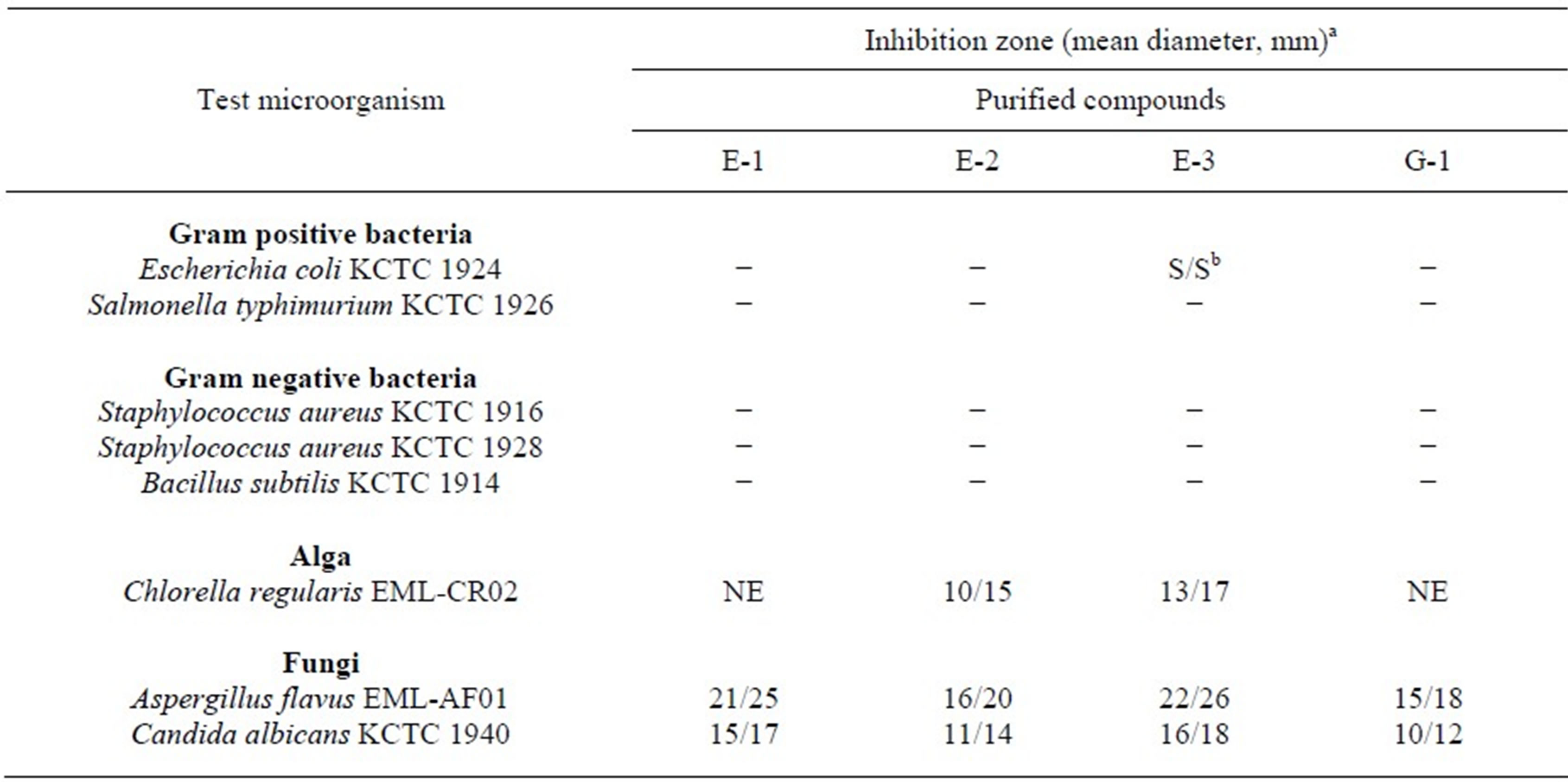
aThe concentrations of the purified EO compounds for microbial inhibition test were 200 µg (left)/500 µg (right) per paper disc (8 mm). bSlight activity. −: No activity. NE: Not examined.
4. Conclusion
In conclusion, the present study clearly indicated that the methanol extract as well as the purified eucosterol oligosaccharides (EOs) from the medicinal plant, S. scilloides, may be used as a natural fungicidal agent or a food preservative for control of molds.
5. Acknowledgements
This study was supported by the Technology Development Program (111095-3) for Agriculture and Forestry, Ministry for Food, Agriculture, Forestry and Fisheries, Republic of Korea.
NOTES