Hematization of Quartz Grains: Study of the Surface of Quartz Grains (M.E.B) and Semi-Quantitative Mineralization ()
1. Introduction
Rock destruction is the main source of soil materials. This phenomenon affects magmatic, metamorphic and sedimentary rocks alike. The purely mechanical actions of erosion agents produce fragments with the same chemical composition as the original rock. Chemical phenomena produce leaching solutions that are exported or provide the elements to form new minerals in the soil. Quartz, a ubiquitous element, is considered an almost unalterable mineral due to its high resistance to weathering. However, previous work shows that this mineral can change its chemical composition in highly oxidized environments (Claisse, 1972; Nahon et al., 1979; Assale, 2013) . According to the work of (Assale & Aka, 2016) , three phases can be distinguished in the Miocene quartz wackes of Bingerville: the quartz phase, the ferruginous phase and the porous phase. This occurs during the dry seasons, when the quartz wackes derived from gresification, under the effect of atmospheric temperature, undergo evaporation of the interstitial water. This promotes the transformation of iron hydroxides into iron oxides through the loss of their water. The geochemical analyses carried out by these authors revealed quartz oxidation. The present work seeks to understand this oxidation phenomenon through the exoscopy of oxidized quartz from the Adiaké locality and its semi-quantitative analysis.
2. Material and Methods
2.1. Presentation of the Study Zone
The quartz samples were taken from the Adia 5 borehole in the Adiaké locality, at a depth of 40m. The quartz grains present are hematoid quartz (Schumann, 2007) composed of yellow or citrine quartz, brown quartz and red quartz. In the 63 μm fraction, the percentage of hematoids decreases to 50% colored quartz and 50% white quartz (Photo 1 and Figure 1). They are mixed with muscovite and oxidized glauconite grains. The age of these formations is Plio-Pleistocene (Assale, 2013) .
For this study, the quartz was sorted with a binocular magnifying glass, then washed with distilled water to remove any ferruginous concretions and clay fractions that might bring extraneous elements to the analysis.
2.2. Semi-Quantitative Mineralogy and Exoscopy of Quartz Grains
These analyses were carried out using the scanning electron microscope (SEM) at PETROCI’s Centre d’Analyses et de Recherche. SEM-EDS gives a semi-quantitative estimate of the weight percentages of major element oxides and the ultrastructure of glauconites and quartz grains. The utrastructure of quartz

Photo 1. Hematoid quartz from Adia 5 borehole.
grains, known as exoscopy, enables us to determine their history and the nature of the source rock. It also enables us to understand quartz inclusion and dissolution phenomena.
Exoscopy consists of identifying the micro-characteristics due to chemical and mechanical actions that may have modified the relief of quartz grains over geological time (Le Ribault, 1977) .
2.3. Statistical Analysis of Chemical Elements
● Graphical representation of chemical elements
The diagrams used for the various graphical representations are histograms and scatter plots. Histograms show the proportions of the various elements present in quartz.
● Correlative analysis
This highlights the various relationships between the chemical elements present in quartz. Correlations are either positive or negative. The closer the correlation (r) is to 1 or −1, the stronger the bond; the closer it is to 0, the weaker or null it becomes.
If the value of r has no intrinsic significance for the interpretation of the relationship, then its square, known as the coefficient of determination, is used. It is interpreted as the proportion of variance of one variable linearly explained by the other variable and vice versa. Proportions of at least 25% will be retained; this is equivalent to r ≥ 0.5.
3. Results
Exoscopy of Adiaké Quartz
The 10 Adiaké quartz grains studied come in two forms: rounded and sub-rounded. The chemical traces observed on the surface of the grains are illustrated in Plate 1 and Plate 2. Photographs were also taken of the grain surface. In Plate 1, the photos show five detailed views of the same rounded grain. All of them show globular iron oxide deposits. The old conchoidal fractures are entirely covered
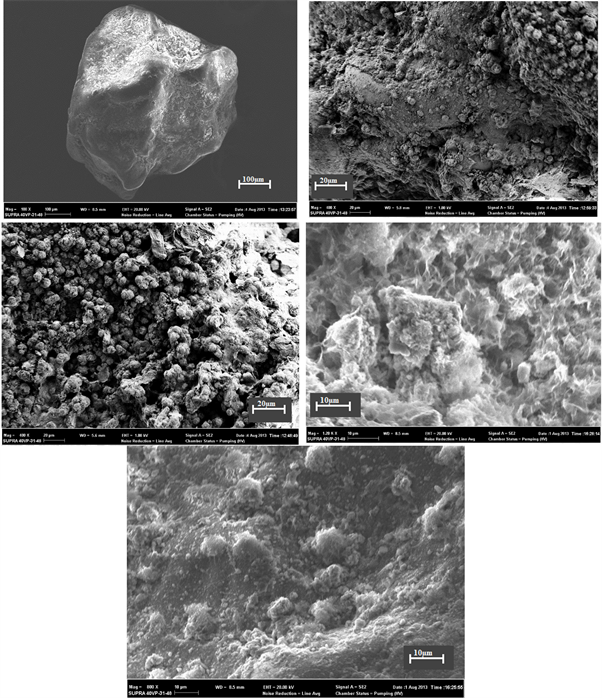
Plate 1. Chemical microstructures observed on quartz from Adia 5 well (40 m).
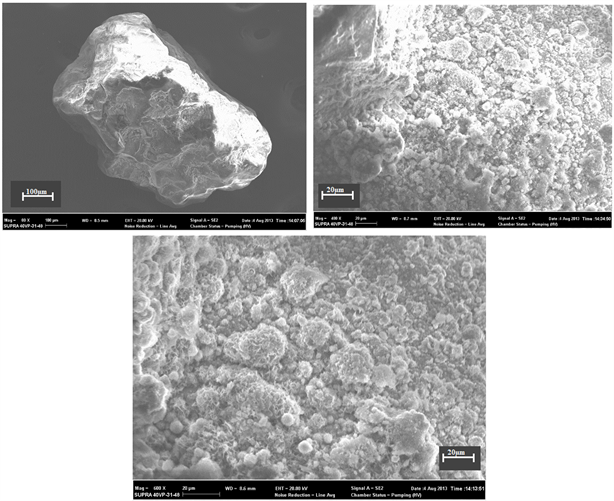
Plate 2. Chemical microstructures observed on quartz from Adia 5 well (47 m).
by a continuous iron film. The other photos are more precise, as they were taken at high magnification.
The traces of chemical and mechanical action are occupied by nanoscopic to microscopic iron nodules, which give quartz its yellow to brown color. It is these traces that promote the hematization of quartz in oxidized environments.
Semi-quantitative mineralogy of hematoid quartz
The results of our analyses are shown in Table 1.
- Graphical representation of chemical elements
The graphical representation of chemical elements shows us that the chemical elements present in quartz after analysis are iron and silicon in large quantities, aluminum and phosphate in medium proportions and all the others in trace amounts (Figure 2).
- Correlative analysis
Negative and positive correlations exist between the oxides Al2O3, SiO2, P2O5 and FeO. Negative correlations concern FeO-SiO2, P2O5-SiO2 and SiO2-Al2O3. They are perfect, good and average respectively (Figure 3). The negative correlations indicate that there is substitution, in order of importance, of silicon by iron at 99.28%, phosphorus and aluminum in these quartz. The brown and yellow colorations are a function of the amount of iron present in the quartz. When the amount of iron is greater than that of silicon, the quartz is brown, and yellow when it is less. Positive correlations are composed of: FeO-P2O5 and FeO-Al2O3 (Figure 4). The positive correlation FeO-P2O5 is good. In contrast, the positive FeO-Al2O3 correlation is poor. The positive correlations show that there is no
![]()
Figure 2. Graphic representation of chemical elements.
![]()
Figure 3. Negative correlation in quartz from Adia 5 borehole (40 m).
![]()
Figure 4. Positive correlation in quartz from Adia 5 borehole (40 m).
![]()
Table 1. Semi-quantitative mineralogy of oxidized quartz.
substitution between the oxides concerned in these quartz. Traces of calcium, sulfur, potassium, dysprosium, terbium and promethium are also found in these samples. These elements are thought to have been acquired during the oxidation of the glauconites that released them. Phosphorus in quartz has a biological origin, from the excrement and/or remains of animals that brought large quantities of phosphates into solution.
In the quartz sedimentation medium, there is a high concentration of iron resulting from the oxidation of glauconites. This indicates that the greater the concentration of iron in a medium, the greater its ability to enter the chemical composition of quartz to form iron oxides such as hematite.
4. Discussion
The exoscopic and semi-quantitative mineralogical studies carried out show that two actions were involved in the transformation of the quartz. The first action occurred during the transport of the quartz, where it underwent fractures. The second occurred during the quartz immobilization phase, when they were subjected to the dissolving action of the environment, creating cavities or corrosions (Kra et al., 2020) . As (Nicolas et al., 2021) point out, the presence of microstructures showing corrosion of the grain surface indicates chemical alteration prior to the appearance of eolian or fluvial transport criteria. These fractures and cavities were then filled by nodules of iron oxide created by the oxidation of glauconite in the medium. This substitution of iron by 99.28% silicon contributed to the coloring of the previously limpid quartz into yellow to brown and then red quartz, which explains the transformation of quartz into hematite. According to (Assale, 2013) exoscopy of quartz grains in the “Bar Earth” shows that it was formed during the last two interglacial periods. These quartz grains underwent dissolution and fragmentation as a result of friction between the grains during transport. Dissolution occurs during the immobile phase and fragmentation during the mobile phase. Dissolution zones may contain mineral inclusions (zircon, quartz, iron, etc.). Moreover, according to (Claisse, 1968) experiments described in a previous study on pure, unaltered quartz showed that quartz is relatively soluble.
5. Conclusion
The semi-quantitative mineralogy and ultrastructure of the quartz grains were studied.
Exoscopy of the quartz grains shows that they have undergone dissolution and fragmentation as a result of friction between the grains during transport. The dissolution zones contain mineral inclusions (zircon, quartz, iron, etc.). The increasing occupation of these zones by iron oxides led to the oxidation of quartz. Quartz that is much more oxidized has a color that tends towards brown.
Semi-quantitative mineralogy reveals the existence of negative and positive correlations between oxides. Negative correlations indicate a substitution, in order of importance, of silicon by iron, phosphorus and aluminum in these quartz. Positive correlations show that there is no substitution between the oxides concerned in these quartz.
Acknowledgements
Our thanks go to the management of PETROCI’s research analysis center (DCAR) and the geology of sedimentary environments and energy laboratory (GESE) of the URF STRM (Earth Sciences and Mining Resources), Université Félix Houphouët Boigny de Cocody Abidjan.