Synthesis, Characterization and Antibacterial Activities of Polydentate Schiff Bases, Based on Salicylaldehyde ()
1. Introduction
Schiff bases are the class of compounds, which are obtained by condensing primary amines and carbonyl compounds [1]. They are also known as an imine or azomethine, is an analogue of a ketone or aldehyde in which the carbonyl group (-C=O) is changed to imine or azomethine (-HC=N-) functionality on reacting with a primary amine (Scheme 1).
Since the discovery of Schiff base [2], it has drawn much attention due to the easy tailoring possibility of the compounds by incorporating different substituents in both amino and aldehydic precursors, which may bring about the variation in the fundamental properties of the synthesized products. The use of Schiff bases in biological or therapeutic applications as promising drug or biological probes has been investigated especially their antibacterial activities [3] [4] [5] [6].
There has been increasing interest on the binding ability of small molecules such as Schiff bases to DNA. Modern coordination chemistry is enriched by Schiff base ligands as metal complexes of Schiff bases are the most widely studied coordination compounds [7]. Increasing importance of Schiff bases as biochemical and analytical reagents are also well documented [8].
Many reports so far on Schiff bases have given rise to several new compounds, and some of them are biologically relevant. The ease with which the Schiff base are designed and prepared have made them to be referred as “fortunate ligands”, with C = N linkage which is relevant for antibacterial, antifungal, antioxidant, anticancer, and diuretic activities [8] [9] [10] [11]. Schiff bases with various donor atoms (like N, O, S, etc.) exhibit broad biological activities and are of special interest due to variety of ways in which they can bind to metal ions, their stability and biological applications [12] [13] [14].
Development of antibiotics against gram-positive and gram-negative bacteria such as Staphylococcus aureus (S.A), Staphylococcus epidermidis (S.E), Klebsiella pneumonia (K.P) and Pseudomonas aeruginosa (P.A) was a challenge, and their invention led successful achievements of modern science by controlling infectious bacterial decreases. Amoxicillin, Norfloxacin, Chloramphenicol, Ciprofloxacin are the most common antibiotics used for these bacterial infections but are associated with side effects such as neurological alterations generated by the interaction of the drug with the central nervous system [15]. Therefore, it is necessary to search for new alternative antibiotics with ease of synthesis and with relatively less side effects [16].
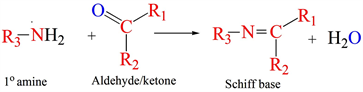
Scheme 1. General synthesis route for Schiff bases. R is -alkyl or -aryl functionality.
Aforementioned facts prompted us to synthesize Schiff bases (L1, L2 and L3) characterize and further investigate for their antibacterial activity.
2. Methods
2.1. Materials
Salicylaldehyde, 2-aminophenol, 4-aminoantipyrine and ethylendiamine were supplied from Aldrich. All solvents were of analytical grade and used without any further purification.
2.2. Physical Methods of Analysis
IR spectra were recorded using KBr discs in the 4000 - 400 cm−1 range on Perkin Elmer IR 65 Spectrometer. 1H NMR measurements were performed on a Bruker Avance 400 MHz using DMSO-d6 as a solvent and TMS as an internal reference. The elemental analyses (C, H and N) were carried out on a Heraeus CHNO rapid analyzer. Agar diffusion method was used to investigate the antibacterial activities by disc diffusion.
2.3. Synthesis of the Compounds
2.3.1. Synthesis of L1
The Schiff base (L1) (Scheme 2) was prepared according to the reported method [17] with modification by the 1:1 ethanolic condensation of 4-aminoantipyrine (2 mmol, 0.4064 g) with salicylaldehyde (2 mmol, 0.2443 g) in 100 ml of solvent. The reaction mixture was then refluxed for 2 h. On cooling, the yellow colored Schiff base was precipitated, which was collected by filtration after washing with diethyl ether and recrystallized from ethanol, which was subsequently dried over P2O5. (Yield = 88.84%).
2.3.2. Synthesis of L2
L2 was synthesized by the synthetic route as described by the reported method [18] with modifications by the Scheme 3. To an ethanolic (100 ml) solution of ethylendiamine (2 mmol, 0.120 g), (4 mmol, 0.977 g) salicylaldehyde was added under stirring. The resultant reaction mixture was then refluxed for 2 h. The Schiff base precipitated as yellow solid, was filtered, washed with diethyl ether and finally recrystallized from ethanol and dried under P2O5 (Yield = 82.90%).
2.3.3. Synthesis of L3
The Schiff base ligand L3 was prepared as described in literature [19] with modified procedure as by the synthetic route shown in Scheme 4. To a stirred ethanolic solution (100 ml) of 2-amiophenol (2 mmol, 0.218 g), (2 mmol, 0.244 g) of salicylaldehyde was added. The reaction mixture was then kept under reflux for 2 h. On cooling, the Schiff base was precipitated filtered, recrystallized from ethanol, dried under vacuum over P2O5 (Yield = 93.60%).
3. Result and Discussions
3.1. Synthesis and Characterization Studies
Analytical studies (Table 1) reveal that the formulations of L1, L2 and L3 are C18H17N3O2, C16H16N2O2 and C13H11N1O2 respectively. L1 and L2 are yellow powders while L3 is dark orange needle shape crystals. The molecular weights of the Schiff bases are shown in Table 1 and the compounds are synthesized in 88% - 92% yield.
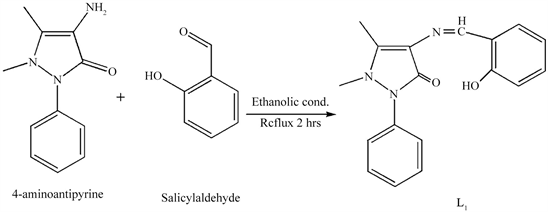
Scheme 2. Synthetic route of L1.
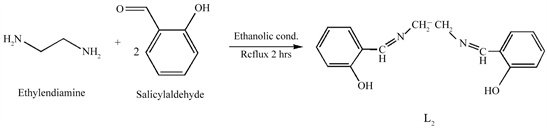
Scheme 3. Synthetic route of L2.
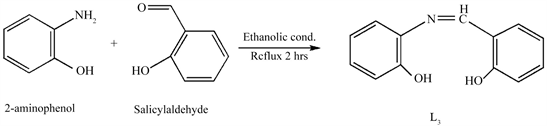
Scheme 4. Synthetic route of L3.
![]()
Table 1. Analytical data of the complexes.
3.2. Spectroscopic Results
3.2.1. FT-IR Spectra of L1
The IR spectra of L1 (Figure 1) and the corresponding assignments (Table 2), reveal that the compound L1 showed a weak broad band at 3505 cm−1, which may be assigned to the ν (OH) vibration. The band at 1596 cm−1 corresponds to νC=N (of azomethine) stretching vibration. The C-O (phenolic) stretching mode appeared at 1362 cm−1. The bands observed at 1676 cm−1, 1505 cm−1 and 1324 cm−1 are stretching vibrations for νC=O, νC=C and νC-N respectively. The peak observed at 1425 cm−1 corresponds to N-CH3 stretching vibration while the aromatic νC-H asymmetric and symmetric stretching bands are observed at 3085 cm−1 and 3075 cm−1 respectively. Additionally, stretching frequency corresponding to νC-H (CH3) appeared at 2945 cm−1 [20] [21] [22].
3.2.2. 1H NMR Spectra of L1
The 1H NMR spectra of the compound L1 is shown in Figure 2 and the corresponding chemical shifts are listed in Table 3. In the spectra, the phenyl multiplets are observed between δ 6.9 - 7.5. The = C-CH3, and -N-CH3 are observed at δ 2.4 and 3.2 ppm respectively. Also azomethine (−CH=N-) resonance is observed at δ 9.7, as a singlet [20] [22] [23].
3.2.3. FT-IR Spectra of L2
The Infrared spectra of L2 are presented in (Figure 3) and the corresponding assignments in Table 2. L2 show a weak broad band at 3499 cm−1, which has assigned to the ν (OH) stretching vibration. Whereas the band at 1605 cm−1 corresponds to the azomethine (νC=N) stretching vibration. The C−O (phenolic) modes of L2 appeared at 1230 cm−1. The bands observed at 1500 cm−1 and 1330 cm−1 are stretching vibrations for νC=C and νC-N respectively. However, the aromatic νC-H asymmetric and symmetric stretching bands observed at 3092 cm−1 and 3005 cm−1 respectively [20] [21] [22].
3.2.4. 1H NMR Spectra of L2
The 1H NMR spectra of the compound L2 and corresponding chemical shift values are presented in Figure 4 and Table 3 respectively. L2 displays phenyl multiplets between 6.8 - 7.4 δ while the -OH and -CH=N signals observed at 13.4 and 8.6 δ respectively as a singlet. In addition to this the -CH2- δ value is observed at 3.9 δ [20] [22].
![]()
Table 2. Summary of characteristic IR absorption (cm−1, KBr) of compounds L1, L2 and L3.
w = weak; m = medium; s = strong.
![]()
Table 3. Summary of 1H NMR spectral data (δ, ppm) of L1, L2 and L3.
sh: sharp; br: broad; s: singlet; m:multiplet.
3.2.5. FT-IR Spectra of L3
The IR spectra of L3 (Figure 5) and the corresponding assignments (Table 2), reveal that the band at 3432 cm−1, corresponds to ν (OH). While the band at 1633 cm−1 is characteristic of the azomethine (C=N), stretching vibration. The band at 1274 cm−1 in the IR-spectrum of L3 is ascribed to the phenolic C-O stretching vibration.
3.2.6. 1H NMR Spectra of L3
The 1H NMR spectra of L3 (Figure 6) and the corresponding chemical shifts (Table 3) show that the δ values between 6.8 - 7.6 δ correspond to phenyl multiplet resonance. The azomethine (-CH=N-) hydrogen resonance is observed at 8.9 δ while the -OH δ values are observed at 9.8 and 13.8 δ [20] [22].
L3 also showed a singlet at δ 9.857 ppm which was attributed to the azomethine (-CH=N-) proton. Also, 1H NMR spectrum of L3 revealed multiplets at 6.8 - 7.6 ppm, corresponding to aromatic protons.
Therefore, it is clear from these results that the data obtained from the elemental analyses; IR and 1H NMR spectral measurements are in agreement with each other.
3.3. Screening for Antimicrobial Activity
The in vitro antimicrobial activity of Schiff base compounds L1, L2 and L3 towards gram positive bacteria Staphylococcus aureus (S.A), Staphylococcus epidermidis (S.E) and gram negative bacteria Klebsiella pneumoniae (K.P) and Pseudomonas aeruginosa (P.A) in Mueller Hinton Agar medium were investigated by disc diffusion method [16]. The solutions of the intended compounds were prepared in methanol at a concentration of 500 μg/ml, 400 μg/ml, 300 μg/ml, 200 μg/ml and 100 μg/ml [24]. At general procedure, 100 μL of the test bacteria were grown in 10 mL of fresh media till they reach a growth of 1 × 108 cells/ml [25]. The microbial suspension was spread onto agar in petridish, which has been maintained in the same condition kept for bacterial growth. Then, methanolic solutions of the test solutions are spotted in the petridish with bacterial growth. It was then incubated for 24 h at 37˚C and then the diameters of the inhibition zones were measured in millimeters (Figure 7).
Standard antibiotics Chloramphenicol and Ciprofloxacin were used as positive control to evaluate the potency of the tested compounds under the same conditions. Activity was determined by measuring the diameter of the zone showing complete inhibition (mm). The same concentration and amount of solvent (methanol) was used as a negative control. Finally the activity results were calculated as a mean ± standard deviation of triplicates.
When compared with the commercially available Ciprofloxacin and Chloramphenicol, the newly synthesized compounds showed appreciable antibacterial activities (Table 4).
The solvent methanol exhibited activity against all bacterial species used with IZs ranging from 8 ± 0.25 to 17 ± 0.29 while the standard antibiotics Ciprofloxacin and Chloramphenicol exhibited high activities with IZs ranging from 21.3 ± 0.31 to 28.3 ± 0.32 and 26.3 ± 0.24 mm to 32.3 ± 0.23 mm, respectively. However, the newly synthesized Schiff base organic compounds L1, L2 and L3 showed IZs ranging from 7.4 ± 0.23 to 32.5 ± 0.14, 3 ± 0.57 to 12 ± 0.28 and 10 ± 0.20 to 32 ± 0.36 respectively.
L3 showed better activity compared to L1 and L2 for all strains of the bacteria studied except S.A for L1. Furthermore, it is interesting to note that the antibacterial activity of L1 and L3 is higher than the activity of both standard antibiotics (Ciprofloxacin and Chloramphenicol) against S.A and S.E respectively, also L3 showed higher activity against P.A than Ciprofloxacin.
Furthermore, the mode of action of the compounds may involve formation of a hydrogen bond through the azomethine group with the active centers of cell constituents, resulting in interference with the normal cell processes. The variation in the effectiveness of the different compounds against different organisms depends on the impermeability of the cells of the microbes or differences in ribosome of microbial cells [24]. Hence it has been inferred that antibacterial activity of the compounds is related to damage cell wall structure of the bacteria, which is essential for the survival of many bacteria and are able to destroy bacteria by inhibiting a step in the synthesis of peptidoglycan layer which is responsible for maintaining the shape of the organism [26].
The MIC is the lowest concentration of the test compound, which inhibits the visible growth of microorganisms after incubation and the MIC is an important diagnostic tool to confirm the resistance of microorganisms towards antimicrobial agents.
The MIC for L3 is 300 μg/ml for S.E and K.P as well as 400 μg/ml for P.A and S.A respectively. However L1 and L2 showed MIC of 100 μg/ml for S.E, S.A and P.A and 200 μg/ml for K.P respectively which is better than L3 (Table 5). In some cases zone overlapping occurs indicating good cleaning action of the tested samples (Figure 7).
![]()
Figure 7. Inhibition zones of L1, L2 and L3 at different concentrations.
![]()
Table 4. Antibacterial activity of ligand, (L1, L2, L3), negative control (Methanol) and positive controls Chloramphenicol and Ciprofloxacin.
![]()
Table 5. MIC. assay of L1, L2 and L3 against bacterial pathogens.
Based on the MIC values presented we can conclude that, the newly synthesized Schiff base compounds L1, L2 and L3 have appreciable MIC when compared with the commercially available antibiotics, Ciprofloxacin and Chloramphenicol with a potency of 5 μg/ml and 30 μg/ml respectively [27].
All the Schiff bases were synthesized by ethanolic condensation reaction and subsequently characterized by elemental analysis, IR and NMR spectra. They are soluble in most polar solvents like methanol and insoluble in almost all non-polar solvents. Based on the analytical and spectroscopic results discussed, the formation of Schiff base compounds has been confirmed. On comparing the antibacterial activities of the synthesized compounds, L3 shows better activity than L1 and L2 against all pathogens, which is higher than the activity of standard antibiotics, Chloramphenicol and Ciprofloxacin for S.E and P.A. Also, it is interesting to note that, all the synthesized compounds exhibited antibacterial activity against all species of bacterium under study, which makes them ideal candidates as antibacterial drugs, after in vivo studies. The major limitations of these synthesized compounds are that in some of the bacterial strains, they showed less activity than the standard antibiotics Ciprofloxacin and Chloramphenicol. However, their antibacterial activity can be improved by tuning their functionality during Schiff base synthesis.
4. Conclusions
Three Schiff base compounds were synthesized, characterized and screened for their antibacterial activity by in-vitro investigations. The antibacterial activities of these compounds were examined using different cultures of bacteria and the results revealed that all the Schiff bases of current study showed appreciable activity.
Among the three compounds, L3 showed the better antibacterial activity for both types of bacteria (gram-positive and gram-negative) as compared to the reference antibiotics Ciprofloxacin and Chloramphenicol. This approach can open new vistas in the chemotherapy of the infectious diseases. The field is further open for pharmacokinetics and clinical trials to establish these molecules as drugs in the market. From the results obtained, we concluded that the newly synthesized compounds could be used as good drug of choice to manage diseases caused by the investigated four bacterial pathogens after evaluating the in-vitro effect on experimental animals and clinical trials. Since all the Schiff bases in the present study show appreciable antibacterial activity, it will be interesting to check the activity of the corresponding metal complexes derived from them, and which is underway. Moreover, the newly synthesized compounds are organic molecules; they may have less side effects to the environment and human being.
Acknowledgements
We are thankful for Addis Ababa Science and Technology University, Ethiopia for financial support by Internal Research Grant AASTU/1006/3311/18.