Wet-Chemical Synthesis and Optical Property of ZnSe Nanowires by Ag2Se-Catalyzed Growth Mechanism ()
1. Introduction
One-dimensional (1D) semiconductor nanomaterials have drawn significant research attention due to their fundamental property versatility and broad applications in electronic and optoelectronic devices [1-3]. As an important II-VI group semiconductor with band gap of 2.70 eV, ZnSe 1D nanostructures, such as nanowires, nanorods and nanobelts, are actively studied in view of their controlled preparation, property engineering and nanodevice fabrication [3-5]. In particular, many efforts are devoted to exploring the controllable synthesis and growth mechanisms of these 1D ZnSe nanostructures with welldefined size, composition and morphology, which is still a challenge of much interest.
In the past decade, many wet-chemical methods have been developed to synthesize 1D ZnSe nanostructures, including solvothermal reaction, colloidal chemistry synthesis and solution-based catalytic route. These wetchemical synthetic methods can be rationally designed in terms of several typical growth mechanisms. For instance, a variety of 1D nanostructures of ZnSe have been effectively prepared through the template mechanism derived from coordination effect in the solvothermal synthesis, where many N-chelating compounds, including hydrazine (N2H4) [6] and organic small molecular amines such as n-butylamine [7], ethylenediamine (en) [8,9], and tetraethylenepentamine (TEPA) [10], are employed as coordination and directing agents. It is even found that some inorganic-organic coordination complexes formed between ZnSe and these N-ligands, such as ZnSe(N2H4)x [6], ZnSe(en)0.5 or ZnSe(en)3 [8,9], and ZnSe(TEPA)0.5 [10], act as metastable 1D soft-templates to produce ZnSe nanorods, nanobelts, nanowires or nanowire-bundles. Meanwhile, the colliodal chemistry synthesis based on surfactants or mixed surfactants, including phosphonic acids (Tri-n-octylphosphine (TOP), tri-nbutylphosphine (TBP)), long carbon-chain amines (n-octadecylamine, oleylamine) and carboxylic acids (oleic acid) [4,11-13], which can lead to the anisotropic growth of ZnSe nanocrystals by modifying the activity of different crystal facets, is effective to grow ZnSe nanorods and nanowires with small size and high uniformity. Another mechanism for the growth of 1D nanostructures is the solution-phase catalytic approaches. One well-known example is the Bi-catalyzed solution-liquid-solid (SLS) regime proposed by Buhro et al., through which ZnSe nanowires of high length and quality are prepared [14- 16]. Recently, a new catalytic mechanism, that is, Ag2Secatalytic growth route, has been explored for preparing ZnSe nanowires [17-19]. This route was successfully performed by our group in the mixed solution of ethanololeic acid or DMF-oleic acid in the presence of surfactants such as polyvinyl pyrrolidone (PVP) and oleylamine [18,19].
In this report, we develop the Ag2Se-catalyzed synthesis of ZnSe nanowires in the mixture solution of oleic acid and oleylamine, which aims to show the good extendibility and generality of Ag2Se-catalyzed mechanism in various reaction media (e.g., solvents, surfactants and reductants). This method is simple, inexpensive, and easy to operate and will be a good complement to the wellknown solution-phase SLS [14-16] and vapor-phase VLS (vapor-liquid-solid) [20,21] catalytic mechanisms for the preparation of ZnSe nanowires.
2. Experimental Section
2.1. Nanowire Synthesis
In a typical synthetic procedure, ZnCl2 (0.2 mmol) and SeO2 (0.2 mmol) were added into the mixed solution of oleic acid (5 mL) and oleylamine (1 mL) in a Teflonlined autoclave of 12 mL capacity. After stirring for several minutes, 0.5 mL of AgNO3 EtOH solution (0.02 mmol/mL) was dropped into the above solution, which is used to produce Ag2Se catalytic seeds by reacting with SeO2. The autoclave containing reaction solution was sealed and kept at 130 - 200˚C for 1 - 3 h. As cooled to room temperature naturally, the dark yellow products were collected by centrifugation, washed three times with ethanol, and then dried in vacuum at 50˚C. ZnSe nanowires could be synthesized at various Ag/Zn molar ratios (calculated by the initially added amounts of AgNO3 and ZnCl2). In this study, the Ag/Zn molar ratio is typically fixed at 5% for the nanowire synthesis.
2.2. Characterization
Room temperature X-ray powder diffraction (XRD) was performed on a Bruker D8 Advance X-ray diffractometer with Cu Kα radiation (λ = 1.54184 Å) at a voltage/current of 40 kV/200mA and a step size of 0.02˚. The XRD pattern was analyzed with a MDI Jade 6.0. Transmission electron microscopy (TEM) and high-resolution TEM (HRTEM) images were performed on a JEOL JEM 2010 TEM at an acceleration voltage of 200 kV, which is equipped with an X-ray energy dispersive spectrometer (EDS, Oxford Inca) for the EDS analysis. UV-Visible diffuse reflectance spectrum was recorded on a Cary 50 UV-Vis spectrophotometer scanning from 200 to 800 nm. The photoluminescence (PL) spectrum was measured using a He–Cd laser with a 325 nm as an exciting source. Both the PL and UV-Vis diffuse reflectance spectra were measured on the as-prepared ZnSe nanowire solid powder at room temperature.
3. Results and Discussion
Transmission electron microscopy (TEM) was used to examine the morphology of the product obtained from the typical synthetic procedure (see Experimental Section). As shown in Figure 1, the product consists of ZnSe nanowires with diameters in the range of 4 - 90 nm and length of from several hundred nanometers to several microns. Interestingly, many of the as-obtained nanowires having a darker particle loaded at their one end are observed, which shows the characteristic feature of nanowire growth in a catalytic mechanism. Our studies (described below) confirm that the darker particle is Ag2Se and the wire is ZnSe. It is also observed that the diameters of nanowires are dependent on the size of the catalytic particles on their tips. Meanwhile, the Ag2Se catalytic particles have a size slightly larger (5 - 13 nm) than the corresponding ZnSe nanowires in the diameter. These growth characteristics of ZnSe nanowires are well consistent with those observed in the SLS or VLS mechanism [14-16,20,21], similar to what we have found for the ZnSe nanowires obtained in the ethanol-oleic acid or DMF-oleic acid mixed reaction system [18,19], except that a broad nanowire diameter distribution is produced in this oleic acid-oleylamine system.
Figure 2(a) shows the room-temperature X-ray diffraction (XRD) pattern of the as-prepared ZnSe nanowires. The XRD pattern can be indexed to the mixed crystalline phases of ZnSe and Ag2Se. In detail, the three strongest diffraction peaks are from cubic zinc blende ZnSe (ZB, JCPDS# 88-2345), matching well with the crystal planes of (111), (220), and (311). The relatively weak peaks marked with asterisk (*) are assigned to hexagonal wurtzite ZnSe (W, JCPDS# 89-2940). Obviously, The ZB ZnSe is dominant in the products, accompanied by a small amount of W polymorph. The XRD peaks present within the blue rectangle are assigned to crystalline Ag2Se, which match well with tetragonal structure of lattice parameters a = b = 7.06 Å and c = 4.98 or 4.76Å [19,22-24]. The tetragonal phase can be seen as another polymorph of Ag2Se, besides cubic and orthorhombic crystal structures [22-24], and has a simple, direct structural transformation relation with the cubic phase (Figure 2(c)) [24]. If the reaction time was shortened from 3 h to 1 h, elemental selenium (Se) appears in the products while the diffractions of ZnSe and Ag2Se are kept unchanged (Figure S1, see Supplement). The yield of Se can be attributed to the reduction of SeO2 by oleylamine.
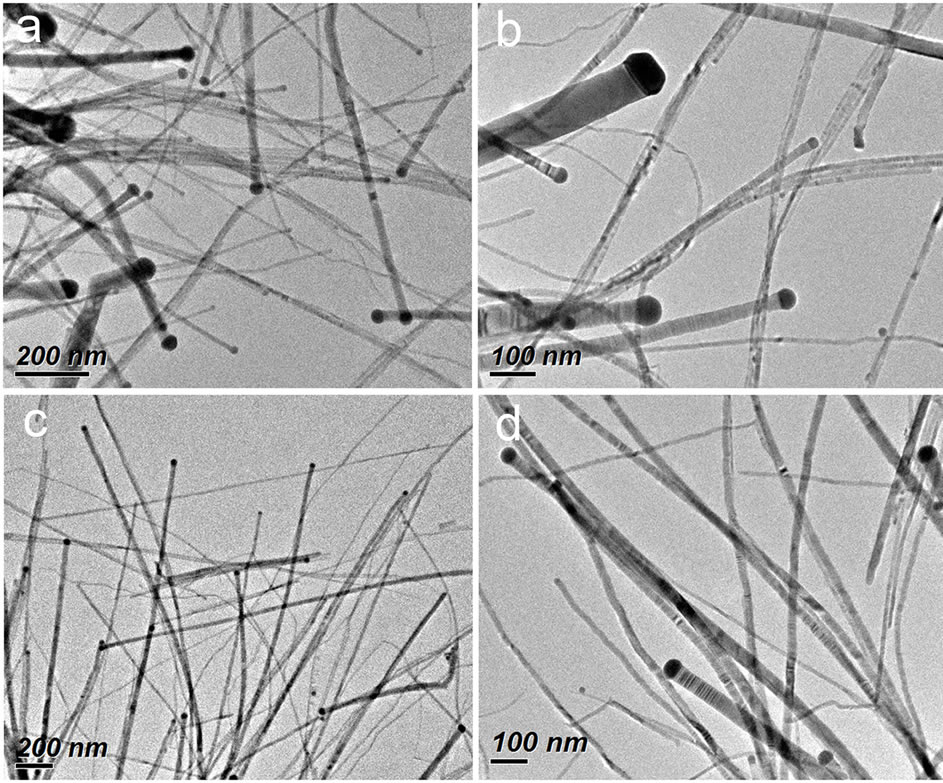
Figure 1. TEM images at different magnifications of ZnSe nanowires prepared from the typical synthetic procedure.
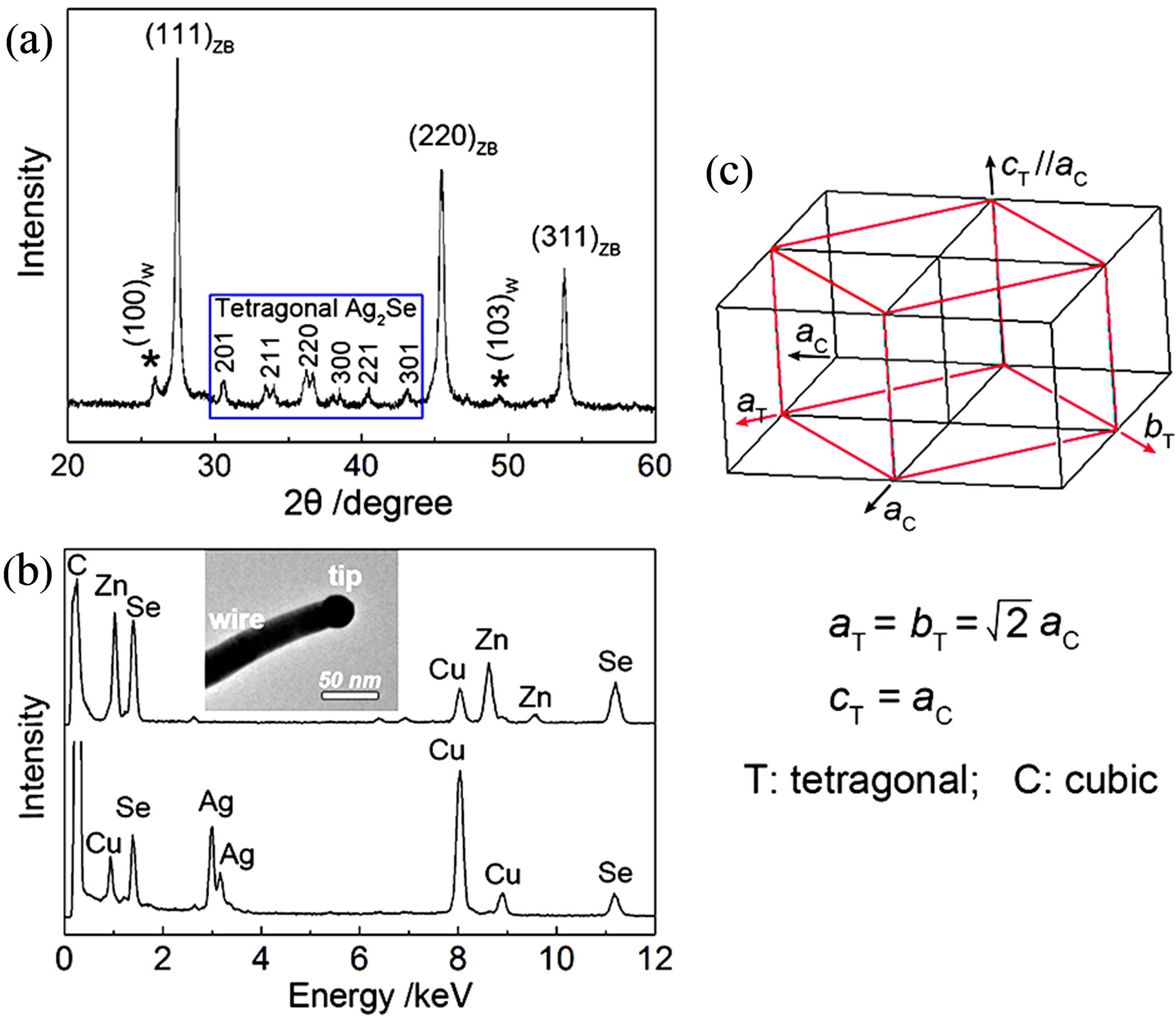
Figure 2. (a) XRD pattern of the as-prepared ZnSe nanowires. (b) EDS spectra recorded on the ZnSe wire and the Ag2Se catalytic tip (the insets). (c) Schematic of the structural relation between cubic and tetragonal Ag2Se.
X-ray energy dispersive spectrum (EDS) was performed to characterize the chemical compositions of the catalytic particle and the wire from a typical Ag2Secatalyzed ZnSe nanowire (the inset of Figure 2(b)). The results reveal that that the dark catalytic particle is composed of Ag and Se with a measured molar ratio of 1.93:1 whereas the wire is of only Zn and Se with a molar ratio of 1.02:1 (Figure 2(b)). Zn signal is not detected in the tip and Ag signal not detected in the wire by EDS analysis within its detection limit. The EDS elemental analyses coupled with the XRD result confirm that the darker catalytic particle is Ag2Se and the lighter wire is ZnSe.
The structural information of Ag2Se catalytic particle and ZnSe nanowire was demonstrated by using highresolution TEM (HRTEM). As shown in the HRTEM image (Figure 3(a)), the well-defined lattice fringes suggest the high crystallinity of both the tip and wire parts. The crystallographic planes corresponding to the lattice fringes for the catalytic tip and the wire can be determined from their interplanar distances and fast Fourier transfer (FFT) patterns. For the wire, the measured distance between two adjacent fringes is 3.29 Å, matching well with the interplanar spacing of the cubic ZB ZnSe (1-11) planes, and the corresponding FFT pattern can be indexed to the [-112] zone axis diffraction pattern, containing another spot of the (220) plane with d = 2.00 Å (Figure 3(b)). These results show that ZnSe nanowires grow along the ZB structure [1-11] direction. As shown in Figure 3(c), the FFT pattern of the catalytic tip can be readily assigned to tetragonal Ag2Se with the incident electron beam parallel to the [010] zone axis, in which the diffraction spots of the (002) and (200) planes are detected with interplanar spacings of 2.48 and 3.54 Å, respectively.
Recently, Connor et al. used Cu2S nanocrystals as seeds to prepare CuInS2 nanorods in solution and they suggested that at the reaction temperature of 250˚C Cu2S nanocrystals are in the superionic conducting state, which promotes the growth of CuInS2 nanorods [25]. As reported in literature, Ag2Se has a first-order solid-solid phase transition at 133 - 135˚C from low-temperature orthorhombic semiconductor phase to high-temperature cubic superionic conductor phase [22-24,26]. In our latest report [19], we confirmed that it is the cubic superionic-phase Ag2Se that catalyzes the growth of ZnSe nanowires in the mixed solution of DMF-oleic acid. In the current work, the used synthetic temperature is 180 - 200˚C and is much higher than the temperature (133 - 135˚C) of phase transition of Ag2Se to superionic cubic phase. So, it is believed that the growth of ZnSe nanowires, performed in the oleic acid and oleylamine mixture solution, is also catalyzed by cubic superionic-phase

Figure 3. (a) HRTEM image of ZnSe nanowires terminated by an Ag2Se particle and the corresponding FFT patterns for (b) ZnSe wire and (c) Ag2Se catalytic tip.
Ag2Se nanoparticles, like the growth of CuInS2 nanorods catalyzed by Cu2S [25]. Interestingly, as determined by XRD and HRTEM analyses (Figures 2(a) and 3), when the reaction temperature is cooled to room temperature the high-temperature cubic phase of Ag2Se catalytic particles is directly converted to the low-temperature tetragonal phase (Figure 2(c)), not the common low-temperature orthorhombic phase [26]. The formation of tetragonal phase Ag2Se is probably due to the small grain size of Ag2Se catalytic particles [22] and the coexisting of ZnSe [27].
The above investigations show that the Ag2Se-catalytic route is an effective method for the synthesis of ZnSe nanowires, where Ag2Se plays the role as Bi in the SLS mechanism [14-16] and Au in the VLS mechanism [20,21]. In the reaction solution of oleic acid and oleylamine, Ag2Se will be precipitated out before ZnSe due to the difference in the Ksp values (Ag2Se << ZnSe), which provides the preexisting catalytic particles for the growth of ZnSe nanowires. Oleylamine is used as a reducing agent in the synthesis and it can easily reduce SeO2 to Se and AgNO3 to Ag [28], which is confirmed our control experiments (Figures S1 and S2). It is considered that Ag2Se is produced from two reaction pathways, the reaction of Se with Ag+ ions and the reaction of Se with Ag, leading to a broad size distribution of Ag2Se catalytic seeds and thus ZnSe nanowires with a large range in the diameters (Figure 1). The control over the uniformity of nanowires is worthy of further study.
Shown in Figure 4 are the UV-Vis diffuse reflectance and photoluminescence (PL) spectra of the as-obtained ZnSe nanowire solid powder. An obvious absorption in the region below 450 nm is detected and the absorption edge (reflectance threshold) is measured at 442 nm, corresponding to a band gap of 2.81 eV. This value is blue shifted by 0.11 eV from that of bulk ZnSe (2.7 eV, 460 nm) [3]. The PL spectrum of ZnSe nanowires shows a

Figure 4. Room temperature UV-vis diffuse reflectance and PL spectra of ZnSe nanowires.
strong, sharp emission peak centered at 414 nm (2.99 eV). To our knowledge, such a significantly blue-shifted PL peak has not reported previously, although this emission is usually related to the near-band-edge (NBE) emission of ZnSe [10,17,29,30]. The reasons for the remarkable blue shift in the UV-Vis absorption and PL emission are not deeply studied and may be the excitation and recombination of excitons trapped by the charged centers at the nanowire surfaces and/or the quantum confinement effect derived from the thin ZnSe nanowires with diameters below Bohr diameter of ~9.0 nm [13]. Meanwhile, a weak broad emission between 500 - 600 nm is also observed, probably due to the Ag-related donor-acceptor pair (DAP) [29,30] luminescence arising from the Ag doping in ZnSe caused by the diffusion of Ag+ ions.
4. Conclusion
In summary, we have extended the wet-chemical catalytic synthesis of ZnSe nanowires in the mixed solution of oleic acid and oleylamine via a catalytic growth mechanism. Ag2Se seeds exhibit excellent catalytic ability for the growth of ZnSe nanowires like Au catalyst in the VLS mechanism and Bi catalyst in the SLS mechanism. The obtained nanowires have been carefully characterized and their UV-Vis diffuse reflectance and PL emission properties are studied. This work provides an alternative catalytic route for synthesizing ZnSe nanowires and would be helpful to the preparation of 1D nanostructures of other metal selenides, which may find their potential use in electronic and/or optoelectronic devices.
5. Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (NSFC, 21201086) and Jiangsu University (11JDG071).
Supplement
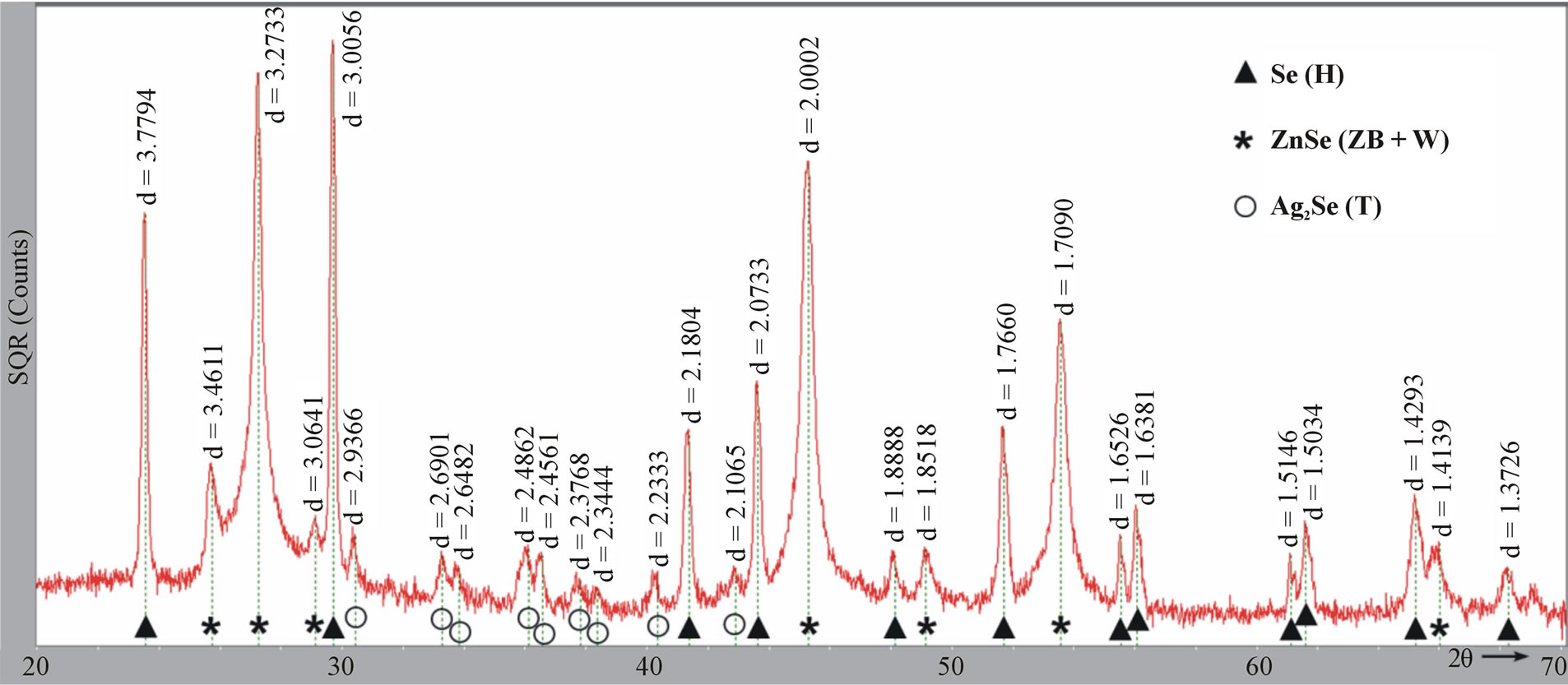
Figure S1.XRD pattern of ZnSe nanowires prepared from 5% Ag/Zn molar ratio at 200˚C for 1 h. The peaks in the pattern match well with diffractions from Se (Hexagonal, JCPDS# 86-2246), ZnSe (ZB + W, JCPDS# 88-2345, 89-2940), and Ag2Se (Tetragonal)

Figure S2.UV-Vis absorption spectrum of Ag nanoparticles reduced by oleylamine in the mixture reaction solution of oleic acid (5 mL) and oleylamine (1 mL) at 180˚C for 20 min. The absorption peak loaded at 403 nm is due to the surface plasmon resonance (SPR) of Ag.
NOTES