One-Pot Process of Anthraquinone Synthesis in the Presence of Mo-V-P Heteropoly Acid Solutions as a Bifunctional Catalysts ()
1. Introduction
9,10-Anthraquinone (AQ) is a quite important product of organic synthesis [1]. Diene synthesis is known as one of industrial processes for producing AQ. This method bases on the 1,4-naphthoquinone (NQ) reaction with 1,3-butadiene [1]. Primary addition products (adducts) are obtained in organic solvents under 1,3-butadiene pressure rising to 0.3 - 2 MPa or in the presence of organic acids allowing to reduce the pressure. Prepared 1,4,4a9atetrahydroAQ (THA) is then oxidized into AQ by strong oxidizers (e.g., CuCl2, H2O2 or NaClO3 [2]) in the acid medium or by air oxygen in the alkaline medium [3].
Earlier [4,5] we suggested to use the aqueous solutions of Mo-V-P heteropoly acids (HPA), which are simultaneously acid catalysts and oxidizers, to produce AQ via 1,4-naphthoquinone reaction with 1,3-butadiene. Using of HPA solutions as bifunctional catalysts allows to carry out the AQ synthesis as a one-pot process (See Scheme 1). At process completion the reduced HPA solution may be filtered away from the solid product and afterwards regenerated, e.g., by oxygen under pressure [6].
In the previous studies [4,5] butadiene reaction with NQ in the HPA solution is shown to yield the product mixtures containing AQ and its partially hydrogenated derivatives THA and 1,4-dihydroAQ (DHA). With this regard, in the present work we tried to optimize the onepot process to obtain practically pure AQ. We have specified conditions, allowing almost complete shift of the adduct oxidation towards the AQ synthesis.
2. Experimental
In all studies we used 1,4-naphthoquinone Alfa Aesar,

Scheme 1. One-pot process of anthraquinone preparation by diene synthesis in Mo-V-P heteropoly acid solutions.
1,3-butadiene (C4H6) of 99% purity. HPA-x (x—the number of V atoms) solutions of different brutto-compositions: Keggin solution of H7PMo8V4O40 (HPA-4) and the high vanadium content non-Keggin solutions H15P4Mo18V7O89 and H17P3Mo16V10O89 (HPA-7 and HPA-10, respectively) were prepared according to method described in [7].
HPLC method was applied for reaction product analysis. For the purpose we used liquid chromatograph ProStar equipped with UV-detector (wavelength 247 nm). Separation was done on column Pursuit 3 C18, 247 × 4.6 mm, eluting reagent flow rate being 1 ml/min. We used commercial available solvents for the chromatography without further purification. Eluting composition was 70% CH3OH + 30% CF3COOH, sample solvent CHCl3.
AQ synthesis was performed in a thermostat autoclave of stainless steel with inserted glass beaker. 15.6 ml of 0.2 M HPA solution and 0.2 g of substrate (NQ) were put into the beaker. If necessary, preliminary NQ was dissolved in the organic solvent. Then autoclave was connected to a butadiene vessel and blown with butadiene to remove air (at required temperature without stirring).
After that autoclave was linked to preliminary heated thermostat, and stirring was put on. All AQ processes were performed under intensive mixing with magnetic stirring (650 min−1) for 1 - 9 hours in the butadiene atmosphere.
Precipitated solid products were filtered, washed with water and dried over P2O5 in vacuum. Additional (less 10%) quantity of product was extracted from the filtrate by chloroform (3 × 30 ml). Thus obtained extract after drying over MgSO4 was evaporated to the dry powder. The combined dry product was weighed, and then exposed to the HPLC analysis. Further in tables it is given the content of combined product.
The NQ conversion was calculated according to formula:

where M is the dry product mass, g; CNQ is the NQ portion in precipitate according to analysis; MNQ—the starting NQ mass, g.
The AQ yield was calculated according to the formula:

where M is the dry product mass, g; CAQ is the AQ portion in precipitate according to analysis; 0.263—the theoretically possible value of AQ mass (for full NQ conversion into AQ), g.
3. Results and Discussion
As we have already mentioned [4,5], when we performed the one-pot process under P = 0.1 MPa and at T = 80˚C - 90˚C without organic solvents or in the presence of solvents poorly mixing with water, we obtained the mixtures of 4 products (traces NQ + THA + DHA + AQ), AQ content never being higher than 50%. To solve problem of maximizing the AQ yield and purity in the onepot synthesis, we tried to optimize its conditions.
The attempt to increase reaction temperature to 135˚C failed to provide essentially higher AQ content. Therefore, our studies were continued at temperatures below 100˚C.
We have assumed reaction mixture heterogeneity to be responsible for the incomplete oxidation of the diene synthesis adducts in the presence of the aqueous HPA solutions. Indeed, NQ is poorly soluble in the HPA solution, while THA and DHA do not solve even on heating. Moreover, the earlier used non polar organic solvents did not mix well with the HPA solutions. Therefore, some organic solvent easily dissolving NQ and easily mixing with water might help the process optimization towards the higher AQ yield. Additional demand to such a solvent was its good resistance to HPA, the latter being a rather strong oxidizer.
In Table 1 one may see the results of experiments with several hydrophilic solvents. Apparently, acetone and 1,4-dioxane provide the product containing mainly AQ and DHA, THA and NQ contents being rather low. Dimethylsulfoxide (DMSO) and especially isopropanol
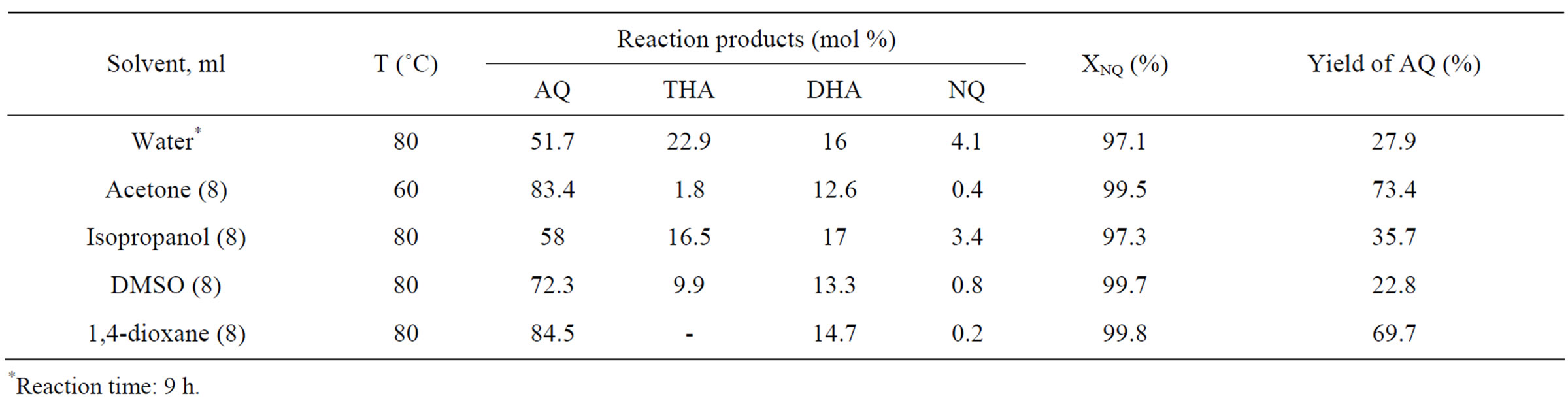
Table 1. Dependence of the AQ synthesis parameters on the organic solvent nature. Reaction conditions: 15.6 ml 0.2 M water solution of HPA-4, molar ratio HPA-4/NQ = 2, butadiene pressure P = 200 kPa—PH2O, reaction time 7 h.
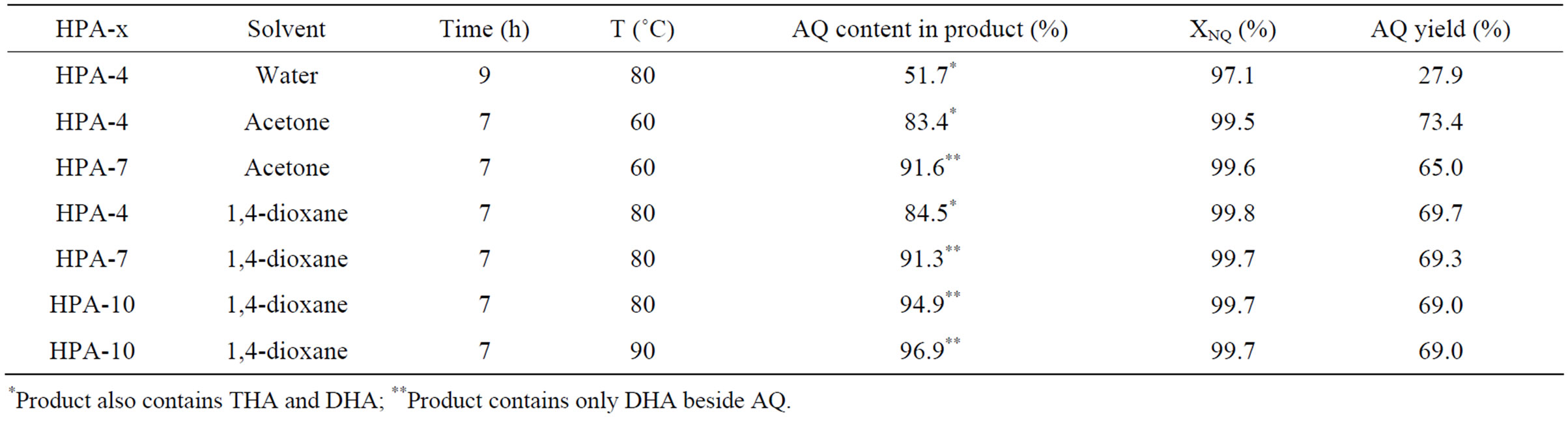
Table 2. AQ synthesis parameters versus the HPA composition. Reaction conditions: 15.6 ml 0.2 M water solution of HPA-x, molar ratio HPA-x/NQ = 2, butadiene pressure P = 200 kPa—P*H2O, organic solvent volume—8 ml.
considerably worsened the final result. The reason most likely lies in the incomplete substrate dissolving in isopropanol. Since there is still some amount of DHA in the product in the presence of above mentioned solvents, we have also tried the HPA-x solutions with high vanadium content (HPA-7 and HPA-10) instead of the HPA-4 solution.
Experimental results are shown in Table 2. As HPA-7 was added to acetone in the course of one-pot process, reaction product contained 91.6% AQ and 8.4% DHA. HPA-10 with dioxane provided 97% AQ (balance DHA), reaction temperature and duration being 90˚C and 7 h, respectively. In this case NQ conversion was 99.7%, and AQ yield was 67%.
Side oxidation processes in the presence of high vanadium content HPA-7 and HPA-10 can account for small total AQ yield decrease in comparison with HPA-4.
Therefore, the simultaneous use of water mixable organic solvent and sufficiently strong oxidizer (HPA-10 solution) allows to obtain AQ having purity up to 97%.
Before catalyst is recovered via reaction (2) by oxygen [8] solvents are distilled from reaction mixture. Thus all solvents are recycled in the following one-pot processes.
4. Conclusions
Therefore, we have optimized the one-pot synthesis conditions to obtain 9,10-anthraquinone via 1,4-naphthoquinone reaction with 1,3-butadiene in the presence of the aqueous solutions of Mo-V-P heteropoly acids. The latter acts as bifunctional catalysts, i.e. acid catalysts in the diene synthesis and redox catalysts, oxidizing the diene synthesis adducts.
It has been shown that it is necessary to use polar water mixable organic solvents (acetone, 1,4-dioxane, etc.) to provide the desired (more than 90%) AQ content in the product and as high as possible the AQ yield (around 70%). The best results were obtained using these organic solvents and Mo-V-P HPA-10 with a high content of vanadium, its brutto composition being H17P3Mo16V10O89.
NOTES