1. Introduction
Multicomponent reactions (MCRs) are highely important reaction for organic chemists, especially in medicinal chemistry feild. [1,2]. Biginelli reaction is one of the most studied reaction in the area of multicomponent reactions [2] and dihydropyrimidin-2(1H)-ones (DHPMs) by the condensation of aldehyde, β-keto ester and urea in the presence of acid [3]. These 3,4-Dihydropyrimidin- 2(1H)-ones are useful in the synthesis of pharmacological activity compounds [4].
Various catalysts have been used to catalyze this reaction like protic acids [5], Lewis acids [6], heterogeneous acidic catalyst [7] and reagents like iodine [8], NBS [9] etc. But many of these protocols are still suffering from different drawbacks such as longer reaction time, high catalyst loading, expensive reagents, corrosive reagents, low yields of products, and large amounts of solid supports which would eventually result in the production of large amounts of toxic waste [10] by considering these facts, we focused our study to task specific ionic liquid and Microwave heating.
The use of ionic liquids (ILs) in chemical research has gained considerable interest from the last decades [11-15]. ILs can be easily modified due to their tunable physicochemical properties according to the needs of the reaction e.g. for specific reactions, extractions or separations, and are then referred to as “task specific ionic liquids” (TSILs) [16].
The use of microwave (MW) irradiation is the alternative heating technique in synthetic chemistry [17]. Various group reviewed ionic liquid/MW combination of different types of organic transformations like cycloaddition reaction, Heck reaction, Aza-Michael addition, Epoxidation, Fisher esterification reaction, Halogenation reactions, Dehalogenation reactions etc [18,19].
Our study is mainly focused on to develop a new and more environmentally benign protocol for Biginelli reaction as well as to synthesize the biologically active compounds using task specific ionic liquids under microwave irradiation [20,21].
2. Experimental Procedure
All the chemicals were purchased from SD fine chemicals, Acros. NMR spectra were recorded on standard Bruker 300WB spectrometer with an Avance console at 300 and 75 MHz for 1H and 13C NMR respectively. All the ILs was prepared according to the procedure reported in literature [6,7,20-22]. The microwave reactions were carried out using BPL Sanyo (India), mono-made, multi power; power source: 230 V, 50 Hz, microwave frequency: 2450 MHz microwave oven.
General procedure for preparation of DHPMs 1-10 A mixture of aldehyde (1 mmol), β-keto ester (1 mmol) and ammonia source (1.5 mmol) were mixed with [Hmim][Tfa] (0.1 mmol) and placed in a 50mL conical flask. The flask was exposed to MW heating for 2 min at 630 W or the whole reaction mixture was heated at 100˚C in a round bottom flask. After the completion the reaction (monitored by TLC: eluent: n-hexane/ethyl acetate: 3/1), cold water was added and the precipitated product was separated by simple filtration. If needed, the crude product was purified by column chromatography or re-crystallization method using ethyl acetate/n-hexane or ethanol as eluent.
3. Results and Discussion
We examined four different types of ionic liquid such as [Hmim][HSO4], [Hmim][Tfa], [Bmim][BF4] and [Bmim] [PF6] for the synthesis of DHPMs under microwave irradiation for our model Biginelli reaction with benzaldehyde, ethylacetoacetate and urea under MW irradiation as well as thermal heating. The results are summaries in Table 1.
As per Table 1, [Hmim][Tfa] was found highly active in term of yield and reaction time under MW heating over conventional heating and the reaction product i.e 3,4-dihydropyrimidin-2 (1H)-ones 4 was obtained in good yield. After getting delightful results, a variety of aromatic aldehydes, 1,3-carbonyl compounds, urea or thiourea underwent three-component condensation smoothly (Scheme 1) were tested and the corresponding reaction products are summarised in Table 2.
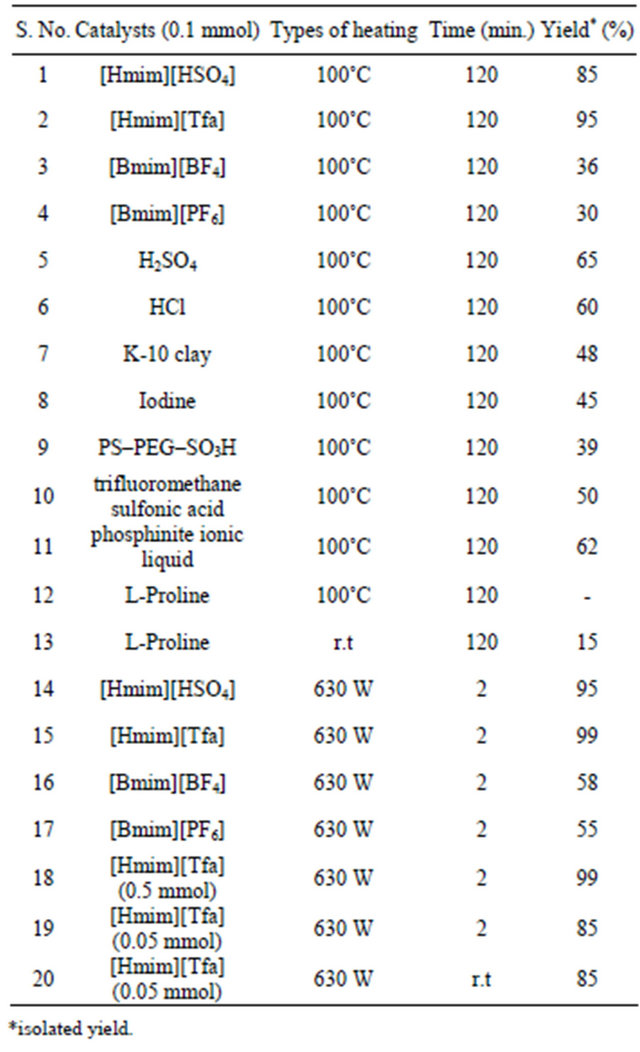
Table 1. Optimization for the reaction condition of Biginelli reaction.

Scheme 1.
The aromatic aldehydes carrying either electron donating or electron-withdrawing substituents reacted well under the reaction conditions to give the corresponding DHPMs in high yield. Thiourea has been also used with similar fashion to produce the corresponding thioderivatives of DHPMs (Table 1, entries 9 and 10), which are also of much interest with respect to their biological activities [21]. Using our development methodology, we have also synthesized the target molecules monestrol and nitractin (Table 2, entries 1 and 8) in high yield (95%) using [Hmim][Tfa] as a catalyst [20]. We have also exploited our proposed methodology for the synthesis of antioxidants (Table 1, Scheme 1, entries 26 and 27) as well as anti-inflammatory via Biginelli reaction, a series of compounds 3-(4,6-disubtituted-2-thioxo-1,2,3,4-tetrahydropy rimidin-5-yl) propanoic acid derivatives (Table 2, entries 29 and 30) were synthesised in high yield and short reaction time.
4. Conclusion
In summary, we coupled task specific [Hmim][Tfa] with MW heating and proposed a simple, active and green protocol for the Biginelli reaction. Using this proposed protocol, we synthesized 30 different biologically active dihydropyrimidinones. Low catalyst loading, reduced reaction time and operational simplicity are the main features of this proposed protocol.