Dissolution of Rare Earth Elements from Coal Fly Ash Particles in a Dilute H2SO4 Solvent ()
1. Introduction
Currently, electricity generated from coal combustion accounts for around 25% of the total power generation in Japan. Since the energy supply, including electricity, is heavily dependent on imported resources, coal occupies an important position in Japan’s electricity generation. Since a reduction in the proportion of electricity generated by nuclear power stations is unavoidable, owing to the disaster at the Fukushima Daiichi nuclear power stations, the utilization of coal-fired thermal electric power stations will become more important.
However, coal-fired power plants are recognized as an emission source of regulated substances such as coal ash; this is a hazardous industrial waste emitted from power plants, and in Japan, 10 Mt of coal ash is emitted per year [1]. Coal fly ash accounts for around 85% - 95% of the total coal ash emission [2] and has been widely recycled and used mainly as a raw material of fly ash cement in order to minimize landfill and to comply with the Basic Act for the Promotion of the Recycling-Oriented Society, enforced since 2000 [3].
Coal fly ash contains various trace elements [4], some of which are recognized to be hazardous. The hazardous inorganic substances include arsenic, boron, cadmium, fluorine, hexavalent chromium, mercury, lead, and selenium; their contents and the elution concentrations are strictly regulated by the Environmental Basic Act and its corresponding legislation [5,6].
Hence, the reduction of the hazardous components eluted from coal fly ash particles is necessary for their recycling. For this purpose, we have developed an acidwashing process [7-9]. The aim of this process is to remove the above-mentioned soluble regulated substances, focused on in our previous studies, from coal fly ash by dissolving them in an acid solvent. We showed that their dissolution, adsorption, and re-elution behavior depends strongly on the pH of the acid solutions and were successful in decreasing elution concentrations below regulatory limits.
In recent years, rare earth elements (REEs) have been recognized as important materials since they are widely utilized in the production of magnets, fluorescent materials, laser crystals, etc. [10]. Since Japan has been the largest consumer of REE products, exploration into alternative supply routes is important for resource security.
Coal fly ash contains relatively high concentrations of REEs. For example, NIST SRM 1633b and IRANT EOP, which are the standard reference materials for coal fly ash, contain 420 mg·kg−1 and 806 mg·kg−1 of REEs, respectively. Although these values are lower than those in ion-adsorbed type ore, they are equivalent to monaziteor bastnaesite-type ore [11].
REEs are known to be soluble in an acidic solvent. Figure 1 shows the Pourbaix diagram for europium, as a typical example of a REE. It is clear that europium is soluble in acid as Eu3+, implying that REEs may be recovered from coal fly ash particles. In this paper, we will discuss the dissolution behavior of REEs in dilute acidic solvents in order to examine the feasibility of REE recovery using an acid-washing process.
2. Materials and Methods
2.1. Coal Fly Ash Specimens Utilized in This Study
Three types of coal fly ash specimens, Ash-A, Ash-B, and IRANT EOP, have been used in this work, and their compositions are listed in Table 1. Ash-A and Ash-B were provided by an electrical power company in Soma City, Fukushima Prefecture, Tohoku Region, Japan. As in the case of general coal fly ash [12], these specimens mainly consist of quartz (SiO2), mullite (3Al2O3·2SiO2), and amorphous solids. The average diameter of the ash specimens was several tens of micrometers. IRANT EOP was obtained as a standard reference material from the Institute of Radioecology and Applied Nuclear Techni-
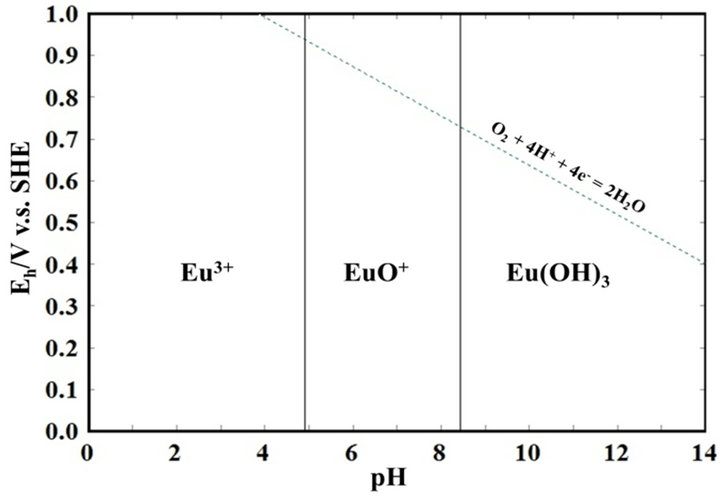
Figure 1. A pourvaix diagram for europium.

Table 1. Chemical compositions of coal fly ash specimens obtained by XRF analysis.
niques, Slovakia, and it has certified concentrations of lanthanum, cerium, and europium, and informative concentrations for scandium, samarium, terbium, ytterbium, lutetium (with expanded uncertainties), dysprosium, gadolinium, neodymium, praseodymium, and yttrium (without expanded uncertainties).
2.2. Reagents and Experimental Procedure
In order to determine the REE concentrations in Ash-A and Ash-B and to verify the accuracy of the measurements obtained by inductively coupled plasma mass spectrometry (ICP-MS), the REE concentrations in the specimens were determined by means of acid digestion. Around 0.1 g of each specimen was put into a Teflon beaker. Reagent grade concentrated hydrochloric acid (35 wt%), nitric acid (60 wt%), and hydrofluoric acid (48 wt%), 10 ml each, were added. Two solutions were prepared for each ash specimen. They were then heated at 200˚C for 6 h on a hot plate. After air-cooling, 10 mL of H2SO4 (95 wt%) were added and heated again to remove any fluorine, originating from the hydrofluoric acid, by vaporization of SiF4(g). After confirming the generation of H2SO4 fumes, indicating the complete vaporization of the fluorine content, they were again air-cooled. At this time, white residual dross, which may be CaSO4, was observed in the solutions of Ash-B. The solutions were moved to 100 mL volumetric flasks, and at this point, the residual dross in the solutions had completely dissolved. After complete digestion, with the dissolution of the white residual dross visually confirmed, the solutions were prepared for measurement with inductively coupled plasma-mass spectrometry (ICP-MS; HP4500, Agilent) using an internal standard technique involving the addition of 0.2 mg·L−1 of rhenium.
The equipment for the dissolution test comprised a Pyrex beaker, a magnetic stirrer, a heater with a stirring magnet, electrodes that were used to monitor the pH, redox potential, and temperature of the solutions, and a laptop computer. Five grams of each coal fly ash specimen were agitated with 500 mL of the H2SO4 solvent, which was diluted at 10 times using ultrapure water. The solutions were agitated at 30˚C, 60˚C, and 80˚C for 2 h. During agitation, the solutions were periodically sampled at 5, 10, 30, 60, and 120 min. The sampling of the solutions was conducted using a 10 mL macropipette. The aliquot was then filtered within 30 s, and 5 mL of each filtrate was diluted to 50 mL in a volumetric flask. ICPMS measurements were then conducted for the solutions with the rhenium internal standard described earlier.
3. Results
3.1. The Determination of REE Elements in Coal Fly Ash
Table 2 summarizes the REE contents in IRANT EOP. The calibration line for the measured REEs was prepared in the range of 1 - 1000 µg L-1. The variance of all calibration lines is 1.00. The values of the REE concentrations are expressed as the mean value ± (2 × standard deviation). Since the true values of the REE concentrations are unknown, the recovery rate was calculated using averaged values as shown in Equation (1).