Neural differentiation of allogenic mixed-cultured rat bone mesenchymal stem cells ()
1. INTRODUCTION
With intensive study to central nervous system (CNS) regeneration, especially with the demonstration of the existence of cells in the developing and adult nervous system that can be isolated and grown in vitro, where they behave as neural stem cells (NSCs), has provided new understanding to neural regeneration and treatment of neural diseases [1]. NSCs are capable of undergoing expansion and differentiating into neurons, astrocytes, and oligodendrocytes in vitro [2,3] and in vivo [4,5], whereas the well-known technical and ethical difficulties of NSCs sources severely limit clinical utility. Therefore, to find a new resource of NSCs is necessary to transplantation therapy of CNS. Many studies have reported that bone mesenchymal stem cells (BMSCs) are capable of transdifferentiating to neural cell types under appropriate experimental conditions in vitro [6-8], BMSCs even could spontaneously differentiate into neural precursor cells after long-term culture [9]. Neurological diseases and CNS trauma have potential loss of neurons. Although NSCs can differentiate into neural cells, repair capacity of neural tissue after injury is limited because NSCs are localized to selected region. Marrow cells are readily accessible, overcoming the risks of obtaining NSCs from the brain. Moreover, BMSCs grow rapidly in culture and not limited by ethical considerations; therefore, it may be useful in the treatment of a wide variety of neurological diseases and CNS trauma. However, neural regeneration needs a lot of cells, sometimes autologous BMSCs can not meet the need, this allows us to consider the utilization of allogenic BMSCs as transplantation cells resouces. The most two frequently used methods for BMSCs isolation are plastic adherence [6,8,9] and density gradient centrifugation [10,11]. Different methods have different defects and virtues. Plastic adherence is an easy method for BMSCs obtaining on the basis of their plastic adherence characteristics, but it is difficult to get pure BMSCs. Cells isolated by density gradient centrifugation method are relatively more pure but with lower reproductive activity. In the present study, we investigated the mixed-culture of allogenic SpragueDawley (SD) rat BMSCs (rBMSCs) isolated by plastic adherence and density gradient centrifugation respectively in vitro, performed immunocytochemistry of nestin, NSE and GFAP, to confirm that mixed-cultured allogenic BMSCs can be induced into neural cell such as neuron and glial cell in vitro.
2. MATERIALS AND METHODS
2.1. Animals
Two male SD rats (6 weeks old, body weight was 93 g and 96 g respectively) were supplied by the Medical Experimental Animal Center, Soochow University, China (license No. SYXK (Su) 2002-0037). Ethical approval was obtained from the Ethical Committee of Soochow University. Protocols were in accordance with the Principles of Laboratory Animal Care (NIH Publication No. 85-23, revised 1985).
2.2. rBMSCs Isolation and Culture
Primary rBMSCs were isolated as previously described by Jendelová [12] and the experience of Jiangsu Institute of Hematology. Briefly, two male SD rats were sacrificed by overdose chloral hydrate injection (1600 mg/kg body weight). Tibias and femurs were dissected free and the proximal and distal ends were removed to reveal the marrow cavity which was aspirated with 10 ml of BMSCs growth medium: low glucose Dulbecco’s modified Eagle’s medium (LG-DMEM) + 10% fetal bovine serum (FBS) (all from Gibco, USA) through a 21 G needle. The aspirate was centrifuged at 1000 rpm for 5 min twice. The cell pellet obtained (containing both haematopoietic cells and BMSCs) was then suspended in BMSCs growth medium. The suspension of one rat was then cultured in four 25 cm2 plastic culture flasks and incubated at 37˚C with 5% humidified carbon dioxide (CO2) for 24 h. Flasks were washed with phosphate buffered saline (PBS) to leave an adherent layer of cells containing rBMSCs. The suspension of another rat was diluted 2:1 with layered over Ficoll (Ficoll-Paque, The Second Reagents Factory of Shanghai, China). After centrifugation at 2000 rpm for 20 min, the mononuclear cell layer was collected from the gradient interface and washed with BMSCs growth medium, then centrifuged at 1000 rpm for 5 min twice. The cells were cultured in two 25 cm2 plastic culture flasks and incubated at 37˚C with 5% humidified CO2. After 48 h, nonadherent cells were removed by replacing the medium.
Fresh culture medium was replaced every 3 - 4 days. When the cultures approached near confluency, the cells were harvested and digested with 0.25% trypsin/1 mM ethylene diamine tetraacetic acid (EDTA) (Gibco) and diluted 1:2 or 1:3 per passage for further expansion. After this cycle was repeated three times, rBMSCs obtained with two different methods were harvested and mixedcultured by 1:1 and repeated this cycle. The second passage of mixed-cultured rBMSCs was obtained to perform Wright-Giemsa staining (fast Wright-Giemsa Stain Kit, Baso, Taiwan, China) for morphological observation: removed medium, washed with PBS, added solution A incubated for 5 min, then added solution B incubated for 5 min, washed with PBS, cleared water with absorbent paper.
2.3. Flow Cytometric Analysis
The second passages of mixed-cultured rBMSCs were characterized by flow cytometry. In brief, cells were trypsinized and washed with fluorescence activated cell sorting (FACS) buffer, consisting of Ca++/Mg++-free PBS, 13.6 mM tri-sodium citrate and 1% bovine serum albumin (BSA) (Sigma, USA). After centrifugation at 2000 rpm for 5 min at 4˚C, cells were suspended in FACS buffer and divided into 4 tubes with 100 μl suspension at a concentration of 1 × 106/100 μl for each antibody tested. Cells were then labeled with 10 μL of fluorescein conjugated monoclonal antibody or standard control at 4˚C for 30 min: the first tube, PE/Cy5 CD29 standard control (BioLegend, USA) and PE CD45 standard control (Caltag, USA); the second tube, PE/Cy5 CD29 (BioLegend) and PE CD45 (Caltag); the third tube, PE/Cy5 CD29 standard control and PE CD90 standard control (eBioscience, USA); the fourth tube, PE/Cy5 CD29 and PE CD90 (eBioscience). The labeled cells were analyzed by flow cytometry within 2 h. At least 20,000 cells for each sample were acquired and analyzed. PE/Cy5 colouration is red, the wave length of excitation light is 488 nm, the maximum wave length is 667 nm; PE colouration is yellow, the wave length of excitation light is 488 nm, the maximum wave length is 578 nm.
2.4. Neural Differentiation
Neural differentiation is according to the experience of Jiangsu Institute of Hematology and Woodbury et al. [6]. In brief, subconfluent mixed-cultured cells were maintained in BMSCs growth medium. Twenty-four hours prior to neural induction, media were replaced with preinduction medium: LG-DMEM + 10% FBS + 1 mM Fibroblast Growth Factor-basic (b-FGF) (Peprotech, Israel). To initiate neural differentiation, the pre-induction media were removed, and the cells were washed with PBS and transferred to neural induction medium: LG-DMEM + 2% Dimethyl sulphoxide (DMSO) (Sigma, USA) + 200 mM butylated hydroxyanisole (BHA) (Sigma).
2.5. Immunocytochemistry
After neural induction for 24 h, media were removed, the mixed-cultured cellse were fixed with 4% (v/v) paraformaldehyde for 30 minutes at room temperature and washed 3 times with PBS, cleared water with absorbent paper. Non-specific binding was blocked by a 40 min treatment with goat serum fluid at 30˚C. The cells were then incubated for 24 h with primary antibody (nestin 1:150; NSE 1:150; GFAP 1:150, all from Boster Bioengineering Limited Company, China) at 4˚C, washed 3 times with PBS, then incubated for 40 min with biotinconjugated second antibody (goat anti-rabbit 1:50) at 37˚C, washed 3 times with PBS, incubated for 30 min with Horseradish Peroxidase-Streptavidin dilution buffer at 37˚C, washed 3 times with PBS, 3,3’-diaminobenzidine (DAB) served as chromagen.
3. RESULTS
3.1. rBMSCs Isolation and Culture
The primary rBMSCs obtained by bone marrow plastic adherence method are uneven in shape, but with excellent reproductive activity. The primary BMSCs obtained by density gradient centrifugation method are fairly uniform in shape, but with lower reproductive activity. After 3 passages, the cells obtained by these two methods both
 (a)
(a) (b)
(b)
Figure 1. Morphology of allogenic mixedcultured BMSCs: cells displayed typical spindle-shaped cells, presented layered and whirlpool-like growth (Wright-Giemsa staining): (a) Magnification ×100; (b) Magnification ×200.
got relative purification. The mixed-cultured BMSCs were good in reproductive activity and relatively uniform in shape. After Wright-Giemsa staining, the mixed-cultured BMSCs displayed typical spindle-shaped cells, presented layered and whirlpool-like growth (Figure 1).
3.2. Flow Cytometric Analysis
Using flow cytometric analysis, the mixed-cultured BMSCs were positive for the surface antigens CD29 and CD90, but negative for CD45 and CD29, CD45, CD90 standard control (Figure 2).
3.3. Neural Differentiation
Before neural induction, there were no obvious changes in the morphology of the mixed-cultured rBMSCs, cells still displayed spindle-shaped cells, presented layered and whirlpool-like growth (Figure 3(a)). While exposure to neural induction medium for 3 h, there were apparent morphological changes in some of the cells, the cells exhibited contracted cell contour with peripheral halo and process-like extensions, changed from flat, elongated, spindle-shaped cells to rounded cells with several branching extensions. After 24 h neural induction, almost all of the mixed-cultured cells displayed neural morphology, with rounded cell body, and their processes formed extensive networks (Figure 3(b)).
3.4. Immunocytochemistry
Immunocytochemistry results showed that induced cells with neural-like morphology were positively stained for nestin, NSE and GFAP (Figure 4).
4. DISCUSSION
According to many previous studies, CD29 and CD90 were regarded as positive cell-surface markers for MSCs, while CD45 was regard as negative surface markers [13- 16]. The mixed-cultured cells used in this study were positive for CD29, CD90 and negative for CD45, indicated that the isolated BMSCs showed typical MSC characteristics.
BMSCs have shown the potential to transdifferentiate into neural lineage under appropriate experimental conditions in vitro [6-8]. The properties of neural differentiation make BMSCs an attractive target for therapeutic applications in neurological diseases and CNS trauma. However, neural regeneration needs a lot of cells, sometimes autologous BMSCs can not meet the need, this allows us to consider the utilization of allogenic BMSCs as transplantation cells resouces. In this study, we investigated the mixed-culture of allogenic SD rat BMSCs in vitro, performed immunocytochemistry of nestin, NSE and GFAP, to confirm that mixed-cultured allogenic
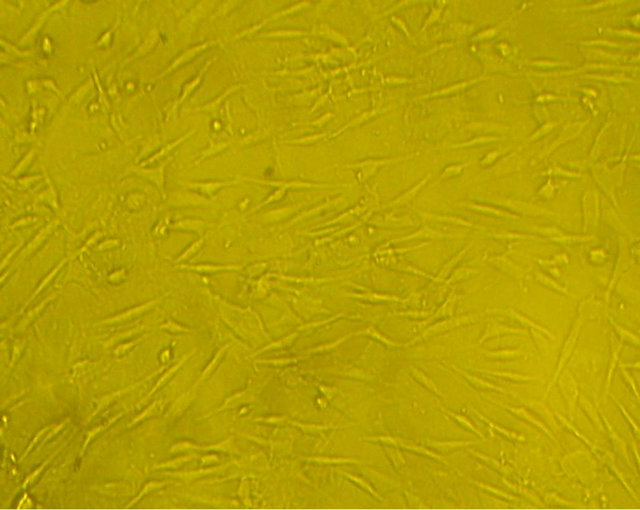 (a)
(a)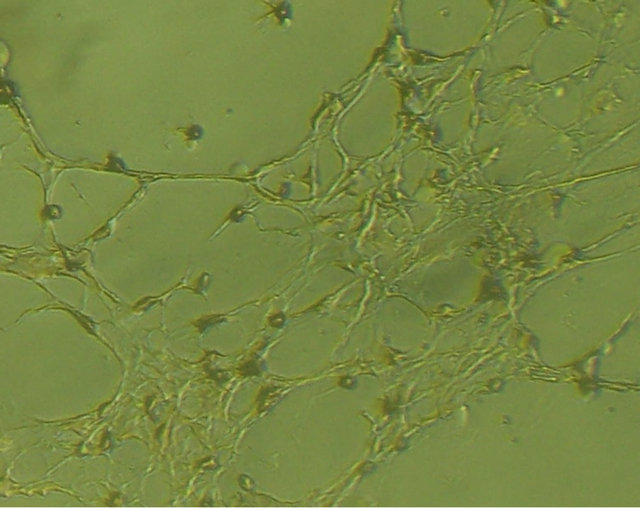 (b)
(b)
Figure 3. morphology of the mixed-cultured rBMSCs: (a) Pre-induction mixed-cultured BMSCs still displayed spindleshaped cells, presented layered and whirlpool-like growth (magnification ×100); (b) Post-induction mixed-cultured BMSCs showed neural morphology, such as rounded cell body and processes formed extensive networks (magnification ×100).
 (a)
(a) (b)
(b)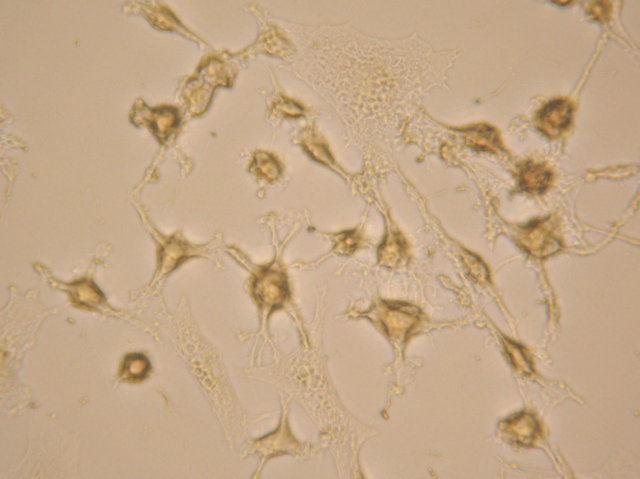 (c)
(c)
Figure 4. After neural induction, mixed-cultured cells showed: (a) Neuronal morphological characteristics, cell bodies became spherical and refractile, nestin (+) (magnification ×200); (b) Processes continued to elaborate, displaying primary and secondary branches, NSE (+) (magnification ×200); and (c) Processes formed extensive networks, GFAP (+) (magnification ×200).
BMSCs can be induced into neural cell such as neuron and glial cell in vitro.
Immunocytochemistry results showed that induced cells with neural-like morphology were positively stained for nestin, NSE and GFAP. nestin belongs to the intermediate filament (class VI) family and is transiently expressed in various progenitors during development, such as neuroectodermal stem cells and the oligodendroglial lineage [17]. During embryogenesis, nestin is expressed in migrating and proliferating cells, whereas in adult tissues, nestin is mainly restricted to areas of regeneration [18]. NSE is a marker of neuron [19], and GFAP is a marker of glial cell [20]. The induced cells positive expressed nestin, NSE and GFAP, clearly demonstrated that mixed-cultured allogenic BMSCs can be induced into neural cell such as neuron and glial cell in vitro.
Woodbury et al. [6] reported that BMSCs undergo transformation into cells with neuron-like phenotypes expressing NSE, neuronal nuclei (NeuN), neurofilament-M (NF-M) and tau. Following treatment is processed with neuronal pre-induction medium consisting of DMEM/ 20% FBS/1 mM β-mercaptoethanol (BME) for 24 h, then treated with neuronal induction medium composed of DMEM/1 - 10 mM BME or DMEM/2% DMSO/200 mM BHA.
Lei et al. [7] use three different methods to induce neural differentiation of BMSCs: BME, serum-free medium and co-cultivation with fetal mouse brain astrocytes. These three methods led to a non-identical results: in serum-free medium, BMSCs mainly differentiated into neuron-like cells, expressing neuronal marker NeuN, and BME can promote this process. After co-cultured with astrocytes, BMSCs leaned to differentiate into GFAP (+) cells.
Tseng et al. [9] observed that after continuous culture for 6 weeks, (32.3 ± 6.3)% BMSCs positive expressed nestin. After cultured in serum-free DMEM/F12 and 20 ng/ml b-FGF for 4 days, these nestin-positive cells expressed neuron-like morphology and neuron-specific markers neurofilament-H (NF-H), beta III-tubulin, tau, and neurotransmitter Gamma-aminobtric acid (GABA). These results demonstrated that neural precursors could be obtained from long-term cultured BMSCs.
These studies made us to reconsider and study whether true neural differentiation of BMSCs takes place following treatment with growth factor and chemical induction medium. According to the experience of Jiangsu Institute of Hematology, Woodbury et al. [6], we determined neural pre-induction medium (LG-DMEM + 10% FBS + 1 mM b-FGF) and neural induction medium (LG-DMEM + 2% DMSO + 200 mM BHA). It is reported that b-FGF promotes transdifferentiation, maintenance of a transdifferentiated state, or cell survival and is involved in the development and maintenance of the nervous system [21]. Tao et al. [22] found that partially differentiated bone marrow cells could not be maintained in culture beyond 3 days post-cytokine withdrawal, suggesting that neuronal differentiation of BMSC under serum-free and feeder cell-free conditions is cytokine dependent. The combination of three growth factors to be crucial: b-FGF to induce neuronal differentiation, epidermal growth factor (EGF) to maintain cell proliferation and differentiation, and plateletderived growth factor (PDGF) to support neuronal differentiation [22]. Both DMSO and BHA are antioxidants, the exact mechanism of them to promote BMSCs differentiate towards neural linage is unclear, possibly related to the trigger of transcripton which determined differentiation direction [23].
Our study revealed that within 3 h of treatment with the induction medium, mixed-cultured cells had neural morphological change. This change was distinguished by rounded and highly refractive cell bodies with neuritelike processes that terminated in structures resembling growth cones and processes formed extensive networks. These findings suggested that the mixed-cultured BMSCs might have differentiated to neural cells. This differentiation potential need appropriate experimental conditions, induction medium may provided these conditions. However, neural-like cells differentiated from mixed-cultured allogenic BMSCs how to possess function of neural cell and the exact mechanism of neural differentiation of mixed-cultured allogenic BMSCs need further study.
5. CONCLUSION
The results of the present study suggest that the mixedcultured allogenic rBMSCs can be induced into neural cell such as neuron and glial cell in vitro.
6. ACKNOWLEDGEMENTS
This study was supported in part by the Natural Science Foundation of Jiangsu Province Youth Fund Project (Grant No. BK2012169), Natural Science Fund for Colleges and Universities in Jiangsu Province (Grant No. 09KJD320007) and Pre-research Project of Soochow University (Grant No. Q3122828). We thank Miss Danhong Shen and Hailan Dai for their help with flow cytometry.
NOTES