Synthesis of Novel 4-Thiazolidinone and Bis-Thiazalidin-4-One Derivatives Derived from 4-Amino-Antipyrine and Evaluated as Inhibition of Purine Metabolism Enzymes by Bacteria ()
1. Introduction
The use of 4-thiazolidinone as chemical fertilizers to increase the yield of crops and pesticides to eliminate all kinds of parasites able to attack the cultivation is becoming more and more important because of the great problem facing the world to provide food to an increasing population [1] . Recently, 4-thiazolidinones derivatives exhibited a wide spectrum of biological medicinal and pharmacological properties such as antibacterial, antioxidant, and hypoglycemic activity [2] . Most of 4-thiazolidinones synthesized exhibited antifungal and antibacterial activity [3] [4] [5] . 4-Amino-antipyrine showed various important properties as an anti-inflammatory [6] , antimicrobial [7] and as an inhibitor of mild steel corrosion in HCl solution [8] . Also, it’s used to eliminate some metal ions as an antifungal agent [9] . Its interest that Schiff’s base analogs of 4-amino-antipyrine exhibited antibacterial and cytotoxic activities [10] . Because of these important results and variables observations, the present work prompted us to synthesize some new Schiff’s bases derived from condensation of 4-amino-antipyrine with aromatic aldehydes followed by cycloaddition with thioacetic acid to obtain the thiazolidin-4-ones in view of their an enzymatic as inhibition of purine metabolism enzymes caused by E. coli. It is known that E. coli strains do not cause disease, but virulent strains can cause gastroenteritis, urinary tract infections, neonatal meningitis, hemorrhagic colitis, and Crohn’s disease. Common signs and symptoms include severe abdominal cramps, diarrhea, hemorrhagic colitis, vomiting, and sometimes fever. Some strains of E. coli, for example, O157:H7, can produce Shiga toxin (classified as a bioterrorism agent). This toxin causes premature destruction of the red blood cells, which then clog the body’s filtering system, the kidneys, causing hemolytic-uremic syndrome (HUS). For these reasons, we focused on synthesize some compounds act as inhibitors for E. coli [11] .
2. Chemistry
The Schiff’s base of 4-amino-antipyrine is a group of systems showed a wide range of biological activities having the azomethine (-N=CH-) active pharmacophore, which plays major roles in their bio-active properties [10] . Also, the presence of cyclic (NCS-C=O) group in thiazolidin-4-ones often enhances those biological and pharmacological properties [12] [13] [14] [15] . Similarly, condensation of 4-aminoantipyrine (1) with selective halogenated aromatic aldehydes and/or heteroaldehydes in refluxing EtOH, yielded the Schiff’s base 2a-f (Scheme 1).
The main aim of the present work is to synthesize of new 4-thiazolidinone bearing antipyrine moiety. Thus, cycloaddition reaction of mercaptoacetic acid with a Schiff’s bases 2a,e,f in refluxing with non-polar solvent as dioxane produced 3-(1'-phenyl-2',3'-dimethyl-5'-oxo-pyrazol-4'-yl) thiazolidin-4-ones (3) (Scheme 1).
Due to the order of nucleophilicity as S > O > N > C, the formation of compounds 3 may be by attack of S− on a more electrophilic carbon of Schiff’s bases 2 followed by elimination of one molecule of H2O (Figure 1).
On other hand, condensation of 4-aminoantipyrine (1) with 1,4-terphthaldehyde (2:1 by mole) in refluxed EtOH, afforded 4,4'-((1,4-phenylenebis (methaneylylidene))bis(azaneylylidene))bis(1,5-dimethyl-2-phenyl-1,2-dihydro-3H-pyrazol-3-one) (4) (Scheme 2).
Similarly, cycloaddition reaction of bis compound 4 with mercaptoacetic acid in refluxing dioxane furnished 2,2'-(1,4-phenylene)bis(3-(1,5-dimethyl-3-oxo-2-phenyl-2,3 -dihydro-1H-pyrazol-4-yl)thiazolidin-4-one) (5) (Scheme 2). Compound 5 also obtained from refluxing of compound 1 with 1,4-terphthaldehyde (2:1 by mole) in excess mercaptoacetic acid in abs. EtOH with a few drops of piperidine for a long time (Scheme 2).
Formation of compound 5 may be as a nucleophilic attack of S− on a more electrophilic center of Schiff base 4 to give the thioacetic acid derivative, which upon refluxing gave the 4-thiazolidinone via losing of H2O (Figure 2).
3. Results and Discussion
Former structure of the new compounds 2-5 established from corrected elemental analysis and their spectral data. The UV spectrum of compound 2a recorded the absorption band at λmax 395 nm, indicated the formation of bio-conjugated systems (N=CH-). FT-IR spectra of Schiff bases 2 exhibited ῡ at 3175 - 3040 & 3035 - 3025 cm−1 for stretching of aromatic CH, with an intense band at ῡ 1680 cm−1 for C=O.
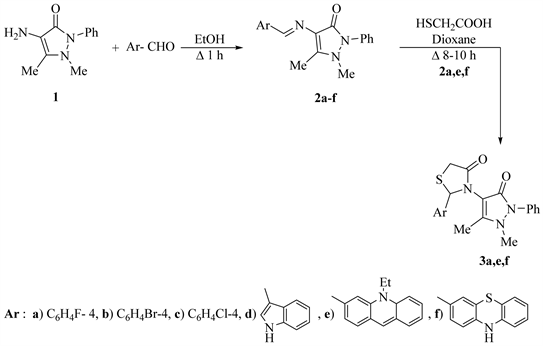
Scheme 1. Synthesis of compounds 2a-f and 3a,e,f.
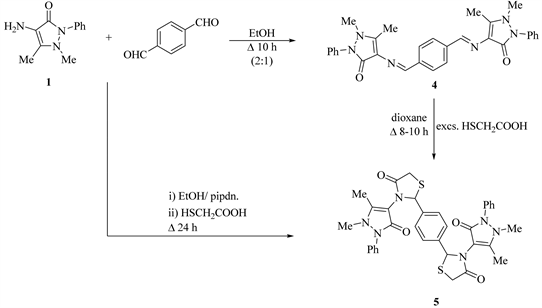
Scheme 2. Synthesis of compounds 4 and 5.
![]()
Figure 1. Formation of compound 3 from 2.
![]()
Figure 2. Formation of compound 5 from 4.
Besides, presence of ῡ at 1590 - 1575 cm−1 for C=N and 2980-2915 cm−1 (stretching modes) with the deformation of aliphatic CH3 at ῡ 1480 - 1440 cm−1.
1H NMR spectra of compound 2a display mainly of two sharp signals at δ 2.25 and 3.0 ppm for N-Me & C-Me protons. Also, it showed δ at 6.80 - 7.90 ppm, 8.2 - 8.5 ppm for aromatic protons with d,d of F-adjacent aromatic protons, besides, δ at 9.4 and 6.3 ppm attribute for CH=N protons. Moreover, mass fragmentation pattern of compound 3a recorded a molecular ion peak with the base peak at m/z 95 attribute to C6H4F ion (Figure 3).
UV absorption spectra of compounds 3 showed λmax 275 nm, lower than the corresponding Schiff’s bases 2. IR spectra of both the compounds 3&5 showed the absorption bands at ῡ at 1720, 1680 cm−1 for true C=O, with lacks of NH groups and or CH=N. 1H NMR spectra of both the compounds 3&5 exhibited a new resonated signal at δ 4 - 3.5 ppm for the presence of CH2 of 4-thiazolidinone with lacks of δ at 9.5 ppm of CH=N, which confirm that structures.
A 13C NMR spectrum of compound 4 recorded a different type of carbons which confirm that structure which gives us a good indication about that structure (Figure 4).
Also, mass fragmentation pattern of compound 4 showed the molecular ion peak at m/e 505 with the base peak at m/z 187 attribute to antipyrine ion (Figure 5 & Figure 6).
In addition, 13C NMR spectrum of compound 5 recorded mainly resonated signals at δ 119 - 118 ppm for cyclic C-S-C with δ at 70 and 170 ppm for CH2 and C=O carbons.
Finally, mass fragmentation pattern of compound 5 recorded the molecular ion peak with the base peak at 187 m/z attribute to 1,5-dimethyl-3-oxo-2-phenylpyrazol ion (Figure 7 & Figure 8).
It is interest that, UV-absorption spectral study showed that λmax of compound 4 is higher than λmax of compound 5, which attribute to inhibition of the conjugated system formed of 4 to an isolated thiazolidinone moiety.
![]()
Figure 3. Mass fragmentation pattern of compound 3a.
![]()
Figure 4. 13C NMR data (δ ppm) of compound 4.
![]()
Figure 5. Mass fragmentation pattern of compound 4.
![]()
Figure 7. Mass fragmentation pattern of compound 5.
4. Biological Inhibition of Purine Metabolism Enzymes
All the synthesized compounds evaluated as enzymatic inhibitors towards purine metabolic by bacteria. The antibacterial effects can be deduced through one of five fundamental mechanisms 1) inhibition of cell-wall synthesis 2) interference with the function of the cytoplasmic membrane 3) inhibition of protein synthesis and 4) interference with cytoplasmic metabolism and final 5) inhibition of nucleic acid fission. A possible mechanism of action is the formation of a type of complexes between different centers, one from the positive microorganisms and the other from the negative drug [16] . According to these finding, the present work aimed to synthesize some new Schiff’s bases and the corresponding thiazolidin-4-ones bearing antipyrine moiety and evaluate their enzymatic properties towards purine metabolic enzymes by bacteria. The new compounds tested at concentrations 32 and 131 μM in the case of E. coli (PNP) and 45 - 65 μM in the case of XAO [17] . Since the analogs Schiff’s base and the corresponding thiazolidin-4-ones, e.q. Allopurinol has therapeutic applications as known as potent inhibitors of the xanthine oxidase (XAO) Enzyme [18] . The IC50 values are given in (Table 1), were insensitive to the concentration of the m7 Guo substrate, indicating a non-competitive type of the inhibition. Enzymatic phosphorolysis of m7 Guo assayed in 50 μM phosphate pH 7.0.
From the results obtained, we can be concluded that the tested compounds have indirect effects on the role of the tested organism. The high IC50 values indicated that the good inhibition toward organism and vice versa low IC50 values give good to moderate inhibition for purine metabolism enzymes.
The order of the inhibitionactivity is as 3a > 4 > 2a > 5 > 3f > 3e. A higher activity of compound 3a may attribute to the presence of F-atom and NCS of thiazolidin-4-one. Also, both compounds 4 and 5 refer to the presence of two thiazolidin-4-ones and two Schiff bases units.
5. Experimental
The melting point recorded on Stuart scientific SMP3 (Bibby, UK) melting point apparatus and reported as uncorrected. A Perkin Elmer (Lambda EZ-2101) double beam spectrophotometer (190 - 1100 nm) used for recording the electronic spectra. A Perkin Elmer model RXI-FT-IR 55,529 cm−1 used for recording the FT-IR spectra. A Brucker advance DPX 400 MHz using TMS as an internal standard for recording the 1H/13C NMR spectra in CDCl3 (δ in ppm). AGC-MS-QP 1000 Ex model used for recording the mass spectra. Elemental analysis performed on Micro Analytical Center of National Reaches Center-Dokki, Cairo, Egypt.
5.1. Schiff’s Bases 2a-2f
A mixture of compound 1 (0.05 mol) and selective aromatic and heteropolyaldehydes (0.05 mol) in abs. EtOH (100 ml) heated under reflux for 1 h, cooled. The solid obtained filtered off and crystallized from suitable solvent to give 2a-2f respectively.
![]()
Table 1. The enzymatic effect of the new compounds on the bacterial (E. coli) purine-nucleoside phosphorylase and xanthine oxidase from buttermilk.
2a. EtOH, yield 83%. M.p: 210˚C - 212˚C. UV (EtOH λmax nm): 320. FT-IR (ATR) ῡ cm−1: 3060, 3040 (aromatic CH), 2950, 2880 (aliphatic CH3), 1670 (C=O), 1580 (C=N), 1200 (bending CH=C), 1250 (C-F). 1 H NMR (CDCl3) δ ppm: 9.40 (s, 1H, -CH=C), 8.20, 8.00 (d,d, H adjacent to F-aromatic), 6.8 - 6.6 (m, 2H, aromatic H), 6.5 - 6.2 (m, 5H, phenyl protons), 2.2, 3.2 (each s, Me-C & Me-N). Anal. Calcd. for C18H16FN3O (309): C, 69.89; H, 5.21; F, 6.14; N, 13.58%. Found: C, 69.45; H, 5.10; F, 6.07; N, 13.38%.
2b. THF, yield 88%. M.p: 227˚C - 229˚C. FT-IR (ATR) ῡ cm−1: 2900, 2880 (aliphatic CH3), 1680 (C=O), 1610 (exo CH=N), 880, 830, 810 (ArH), 780 (C-Br). Anal. Calcd. for C18H16BrN3O (369): C, 58.39; H, 4.36; Br, 21.58; N, 11.35%. Found: C, 58.31; H, 4.26; Br, 21.21; N, 10.99%.
2c. EtOH, yield 87%. M.p: 128˚C - 130˚C. FT-IR (ATR) ῡ cm−1: 2980, 2870 (aliphatic CH3), 1670 (C=O), 1620 (exo CH=N), 870, 830 (ArH), 700 (C-Cl). Anal. Calcd. for C18H16ClN3O (325): C, 66.36; H, 4.95; Cl, 10.88; N, 12.90%. Found: C, 65.99; H, 4.82; Cl, 10.82; N, 12.73%.
2d. MeOH, yield 88%. M.p: 253˚C - 255˚C. FT-IR (ATR) ῡ cm−1: 2960, 2880 (aliphatic CH3), 1680 (C=O), 1610 (exo CH=N), 910, 860, 830 (ArH). Anal. Calcd for C20H18N4O (330): C, 72.71; H, 5.49; N, 16.96%. Found: C, 72.68; H, 5.38; N, 16.77%.
2e. MeOH, yield 89%. M.p: 196˚C - 198˚C. FT-IR (ATR) ῡ cm−1: 2970, 2880 (aliphatic CH3), 1680 (C=O), 1620 (exo CH=N), 820, 810 (ArH). Anal. Calcd. for C27H26N4O (422): C, 76.75: H, 6.20: N, 13.26 %. Found: C, 76.45; H, 5.99; N, 13.15 %.
2f. THF, yield 85%. M.p: 165˚C - 167˚C. FT-IR (ATR) ῡ cm−1: 2975, 2890 (aliphatic CH3), 1678 (C=O), 1618 (exo CH=N), 1200 - 1100 (C-S-C), 834, 810 (ArH). Anal. Calcd. for C24H20N4SO (412): C, 69.88; H, 4.89; N, 13.58; S, 7.77%. Found: C, 69.81; H, 4.80; N, 13.29; S, 7.55%.
5.2. 3-(1'-Phenyl-2',3'-Dimethyl-5'-Oxo-Pyrazol-4'-yl)- Thiazolidin-4-Ones (3a,3e,3f)
A mixture of 2a, 2e & 2f (0.01 mol) and mercaptoacetic acid (0.15 mol) in dioxane (70 ml) heated under reflux for 6 - 8 h, cooled, then neutralized with eq. NaHCO3. The solid produced filtered off and crystalized from suitable solvent to give yellowish crystals 3a, 3e & 3f respectively.
3a. Dioxane, yield 60%: M.p: 185˚C - 187˚C. UV (λmax nm) 275. FT-IR (ATR) ῡ cm−1: 3060, 3040 (aromatic CH), 2950, 2840 (aliphatic CH), 1700, 1670 (2C=O), 1250 (C-F), 1190 (C-S-C), 700 (C-F). 1H NMR (CDCl3) δ ppm: 8.2, 8.0, 6.8 - 6.4 (m, 9H, aromatic protons), 4.8 (s, 1H, exo), 4.0 (m, 2H, CH2), 3.1 (s, 3H, N-Me), 2.3 (s, 3H, C-Me). 13C NMR (CDCl3) δ ppm: 172 (C=O), 158 (C=O), 135 (C=C), 130 - 122 (aromatic carbons), 125 (C-F). 118 (C-S-C), 62 (CH2), 40, 38 (-C-N, -C-C). M/S (m/e, Int. %): 383 (0.15), 196 (8.75), 187 (13.25), 120 (25.19), 95 (100), 67 (5.11). Anal. Calcd. for C20H18FN3O2S (383): C, 62.65; H, 4.73; F, 4.95; N, 10.96; S, 8.36%. Found, C, 62.49; H, 4.55; F, 4.81; N, 10.79; S, 8.11%.
3e. EtOH: yield 55%, M.p: 210˚C - 212˚C. FT-IR (ATR) ῡ cm−1: 2982, 2876 (aliphatic CH3), 1710, 1678 (2C=O), 1200 - 1180 (C-S-C), 912, 820 (ArH). Anal. Calcd. for C29H28N4O2S (496): C, 70.14; H, 5.68; N, 11.28; S, 6.46%. Found: C, 70.01; H, 5.12; N, 11.01; S, 6.29%.
3f. MeOH, yield 50%, M.p: 180˚C - 182˚C. FT-IR (ATR) ῡ cm−1: 2980, 2880 (aliphatic CH3), 1700, 1675 (2C=O), 1200 - 1190 (C-S-C), 880, 830, 810 (ArH). Anal. Calcd. for C26H22N4O2S2 (486): C, 64.18; H, 4.56; N, 11.51; S, 13.18%. Found: C, 63.95; H, 4.12; N, 11.33; S, 12.98%.
5.3. 4,4'-((1,4-Phenylenebis(Methaneylylidene)) Bis(Azaneylylidene))Bis(1,5-Dimethyl-2-Phenyl-1,2- Dihydro-3H-Pyrazol-3-One) (4)
Compound 1 (0.02 mol) and terphthaldehyde (0.01 mol) in abs. EtOH (50 ml) warmed for 30 min, then cooled. The solid produced filtered off and crystallized from AcOH to give 4, yield 90%, M.p: 222˚C - 224˚C. UV (EtOH λmax nm) 396. FT-IR (ATR) ῡ cm−1: 3175 - 3040, 3035 - 3025 (aromatic CH), 1655 (azomethine HC=N), 1650 (C=O), 1590 - 1575 (C=N), 2980, 2915 (str. Aliphatic CH3), 1480 - 1440 (bending CH3). 1H NMR (CDCl3) δ ppm: 9.40 (s, 1H, CH=N), 7.90 - 6.80 (m, 14H, aromatic CH), 3.00 (s, 3H, N-Me), 2.25 (s, 3H, C-Me). 13C NMR (CDCl3) δ ppm: 172 (C=O), 140 (CH=N), 135 (C=C of pyrazole), 130 - 120 (aromatic carbons) 45 (Me-N), 40 (Me-C). M/S (m/z, Int. %): 505 (M+1, 1.01), 187 (100), 120 (13.25), 76 (38.7), 67 (0.55), 52 (45.00). Anal. calcd. for C30H28N6O2 (504): C, 71.41; H, 5.59; N, 16.66%. Found, C, 70.96; H, 5.48; N, 16.47%.
5.4. 2,2'-(1,4-Phenylene)Bis(3-(1,5-Dimethyl-3-Oxo-2-Phenyl-2,3- Dihydro-1H-Pyrazol-4-yl)Thiazolidin-4-One) (5)
Compound 4 (0.01 mol), mercaptoacetic acid (0.04 mol) in dioxane (50 ml) heated under reflux for 8 - 10 h, cooled then neutralized by aq. NaHCO3. The solid obtained filtered off and crystallized from MeOH, to give 5, yield 66%, M.p: 275˚C - 277˚C. FT-IR (ATR) ῡ cm−1: 3080, 3040 (aromatic CH), 2910, 2850 (aliphatic CH), 1700, 1660 (2C=O), 1480, 1440 (deform. CH2), 1190 (C-S-C), 880, 820 (aromatic rings). 13C NMR (CDCl3) δ ppm: 172, 162 (2C=O), 132 (C=C), 130 - 126 (aromatic carbons), 119 (C-S-C). 70 (CH2), 40 & 38 (Me-N & Me-C). M/S (m/z, Int %): 654 (0.11), 187 (100), 176 (1.5), 159 (2.11), 101 (5.11), 76 (80.13), 74 (1.2). Anal. Calcd. for C34H32N6O4S2 (652): C, 62.56; H, 4.94; N, 12.87; S, 9.82%. Found: C, 62.21; H, 4.63; N, 12.59; S, 9.74%.
5.5. Formation of Bis-Compound 5
A mixture of compound 1 (0.02 mol), 1,4-terphthaldehyde (0.01 mol), and excess of mercaptoacetic acid in EtOH/ drops piperidine heated under reflux for 24 h, cooled. Then treated with aq. K2CO3 followed by addition of drops of HCl. The solid obtained, filtered off and crystallized from EtOH to give 5. Melting point of both methods and a mixed melting point not changed.
6. Conclusion
Some new 4-thiazolidinones and bis-compounds have been synthesized and derived from condensation of 4-aminoantipyrine with aromatic aldehyde followed by cycloaddition of thioglycolic acid in a non-polar solvent. Fluorine substituted thiazolidin-4-one moiety bearing antipyrine nucleus enhanced the enzymatic effects of the bacteria, which agreed with last results published [19] [20] [21] .
Acknowledgements
Many thanks for King Abdulaziz University, Jeddah, SA.