Immunological Manifestations in Paraphenylene Diamine Poisoning ()
1. Introduction
Paraphenylene diamine (PPD) or para-aminobenzene is an aromatic amine derivative of aniline, used since 1863 by women for cosmetic purposes like black hair dye or mixed with “henna” (leaves of Lawsonia alba) in several african countries and middle east, because PPD accelerates the dyeing process. This chemical is widely known in western industry by many names (paramine, fouramine D, ursol D, vulpa D, furol S) and used in several industries: dyeing furs, oxidisable hair dye, tyre vulcanization industries and photochemical processes, etc. [1]. In Morocco, it is freely sold by traditional herbalists as the “Takaout Roumia” by analogy to a vegetal nontoxic product derived from scab of Tamaris orientalis named “Takaout Beldia”. The widespread use of PPD and its availability in the absence of regulation of its use led to the discovery of its toxic effects, hence its frequent use in order to autolysis [2]. Acute PPD poisoning causes severe edema of the face and neck frequently requiring emergency tracheotomy. This is followed by rhabdomyolysis and acute renal failure, culminating in death if not treated aggressively [3]. Some clinical studies have been published to document the acute toxicity of PPD [2-9]. The classic physiopathologic approach of the manifestations after the intoxication by the paraphenylene diamine (PPD) presents many limits. Recently, the immunogenic role of the PPD (particularly its derivatives of oxidation) in the genesis of contact dermatitis and immunologic perturbations have been revealed. Our aim is to establish the immunologic profile of the PPD-intoxicated persons based on a monitoring of the inflammatory reaction.
2. Patients and Methods
It was a prospective observational study including all PPD-intoxicated patients, realized in the medical intensive care unit in university hospital of Casablanca during 2010. We evaluated demographic and clinical parameters, and biological data: white blood cells, the protein-Creactive, the fractions C3 and C4 of complement and lymphocytes subpopulations CD3, CD4, CD8 and CD19. A follow-up was realized and the kinetic of laboratory data was compared with clinical findings.
3. Results
A total of 21 patients with PPD poisoning had been recruited in our study. The median of age was 20 years [15 - 52]. The sex-ratio (F/M) was 20%. The intoxication was voluntarily aiming at autolysis in 100% of the cases. The patients were admitted at about 3.5 hours after the intoxication. The mean length of stay in ICU was 16 ± 4 days [2 - 75]. The evolution of the median rate of white blood cells (/mm3) was detailed in Figure 1, the median value of C-reactive protein (mg/l) in Figure 2, the median value of C3 and C4 (g/l) of complement in Figure 3, and the median value of lymphocytes subpopulations (/mm3): CD3, CD4, CD8 and CD19 in Figure 4.
4. Discussion
The PPD has many names: 1,4-Diaminobenzene; 1,4- Benzenediamine; 4-Aminoaniline; para-Diaminobenzene; para-Benzenediamine and para-Aminoaniline [10]. The acute toxicity has been investigated following oral, subcutaneous, intraperitoneal and topical application in a variety of species. The LD50 following oral administration was 80 mg/kg - 100 mg/kg in the rat, 290 mg/kg in mice, 250 mg/kg in rabbit and 100 mg/kg in cats [11,12]. Benzene, benzoquinone and hydroquinone are PPD metabolites.

Figure 1. Evolution of the median rate of white blood cells (/mm3).

Figure 2. Evolution of the median value of C-reactive protein (mg/l).

Figure 3. Evolution of the median value of C3 (g/l) and C4 (g/l) of complement.
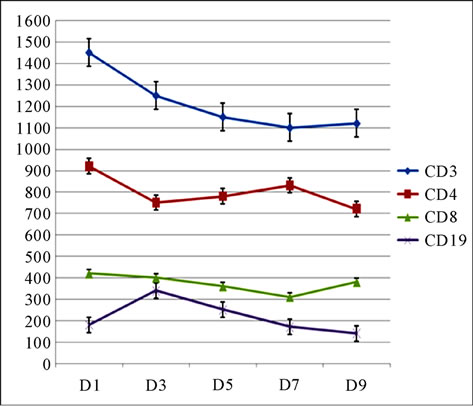
Figure 4. Evolution of the median value of lymphocytes subpopulations (/mm3): CD3, CD4, CD8 and CD19.
The effects of PPD and its metabolites on the immune system are not well known. Ewes et al. declared that up to a dose of 100 nmol p-benzoquinone/mouse the absolute numbers of B and T cells were increased and that the increase in B cells was higher than that in T cells. Absolute numbers of CD4+ and CD8+ cells also increased, but the ratio CD4+/CD8+ did not change [10]. The same result was showed by Coulter, that the exposure to PPD is was associated with 1) the stimulation of CD4+ and CD8+ T cells and 2) significantly higher levels of Th2 cytokine secretion and increased gene expression [13]. MacEachern and Laskin reported nonspecific immunotoxic effects by modulation of cytokine production in bone marrow leukocytes of benzene-exposed mice [14]. Recently, Lee [15] suggested that hydroquinone acts as a strong inhibitor of activated macrophages. It suppresses the production of proinflammatory cytokines, secretion of cytotoxic molecules, and the expression and activation of CD29. PPD could also induce apoptosis via the involvement of reactive oxygen species [16].
The monitoring of the inflammatory and immunological reactions of our patients permits to describe two evolutionary phases which can be correlated with the clinic evolution.
The first phase (from day 0 (D0) to day 6 (D6)) is characterized by the association of an “immunological paralysis” and rhabdomyolysis, considered as a pro-inflammatory phenomenon. In this phase we can distinguish two steps: the first one (D0 - D3), with a dominant inflammatory stress, is characterized by 1) a leucosis 2) an increase rate of CRP, 3) a relative decrease of C3 and C4 fractions of the complement, rate of lymphocytes, CD3 and CD4 but always in the normal interval, 4) and by low rates of the CD8 and the CD19; the second one (from D3), is characterized by a first blood pick of leukocytes and CRP, an increasing C3 and C4 rates and by a relative increase of CD4 rate revealing a stress-end. The rate of CD8 and CD19 remains always low.
The second phase, from D7, corresponded on the immune response to PPD and her oxidative metabolites. These results explain the secondary clinical aggravation of our patients (shock, ARDS, DIVC…).
Experimentally, the PPD provoke a retarded inflammatory reaction in mice (from D6 after exposition) [10]. Our results are similar with this report, from D7, the monitoring of inflammatory reaction showed the following perturbations that only the rhabdomyolysis could not explain. Indeed, 1) the leucosis reaches its second pick (D6) and continue to evolve in tray; 2) the CRP reaches the maximal pick on D6, evolve in tray during two days before starting a decrease; 3) relative increase of rates of total lymphocytes and CD3; CD4 blood pick on D7, then relative decrease; with relative increase of the rates of the CD8 revealing the cytotoxic cellular character of this inflammatory reaction and the rate of the CD19 continues to lower.
Therefore, the monitoring of the inflammatory reaction in our patients explained the clinical evolution in three times of this reaction. In the first time, inflammatory stress (during the three first days after the intoxication) is characterized by a relative immunodepression. In the second time (from the 3rd day) rhabdomyolysis exerts proinflammatory power. A third time (from the 6th day) corresponded to immunomodulative action of PPD and to its oxidative metabolism. It is a systemic inflammatory reaction specific to a cytotoxic cell support, this time would explain the secondary worsening of the clinic and paraclinic parameters of our patients (hemodynamic shock, multivisceral failures, etc.). This observation is reported by Richter who suggests that environmental chemical stress and agents can reduce the resistance of children to bacterial and viral infections [17].
Finally, it seems that the immunological aspect may present the answer to several questions to which the PPD-rhabdomyolysis alone couldn’t answer. This study tried to establish a first immunologic profile of the intoxicated persons with the PPD, and to correlate it with their clinical evolution.