Received 15 April 2016; accepted 16 August 2016; published 19 August 2016

1. Introduction
Zea mays L. belongs to the grain-producing family―Poaceae [1] . The name maize was derived from South American Indian Arawak-Carib word called-Mahiz. This seed is also called corn by the British and Americans which means “that which sustains life” [2] . Maize is a widely adopted crop capable of production during the appropriate season in almost all parts of the world where farming is done. According to Fatima and Abdul [3] , the major cereals grown in the sub-savanna region of Nigeria include: maize (Zea mays L.), sorghum (Sorghum bicolor L.) and rice (Oryza sativa L.). In Nigeria, maize was first introduced probably in the 16th century by the Portuguese. Global production of maize was estimated to be around 800 million tons in 2007, slightly more than rice which was 650 million tons [4] . Mycotoxin contaminations of grains, especially those produced by species of Aspergillus render a significant portion of the agricultural produce in the world unfit for human consumption [5] . Over 300 fungal metabolites have been reported toxic to man and animals and more than 25% of the world cereals are contaminated with known mycotoxin [6] .
A major cause of plant diseases is fungi and is responsible for large scale harvest failures in crops like maize and other cereals all over the world [7] . Cultivation of maize is limited by diseases which cause grain loss of about 11% of the total production. The most widespread infection in stored grains is caused by fungi and they appear as mold or caking on the affected ears or grains. The corn loses colour, viability and reduction in food value. The poisonous mycotoxin is the most dreaded by-product of fungal attack. Mycotoxins cause poisoning in both livestock and human [8] . Uzma and Shahida [9] reported that more than sixty diseases affect maize. Several kinds of fungi can be associated with maize grains during storage either causing their deterioration or simply remaining viable to infect germinating seedlings. Aspergillus, Penicillium, Fusarium and some xerophytic species are fungi genera typically found in stored grains and several of them with capabilities of producing toxins [10] . Some fungi especially species of Aspergillus, Diplodia, Penicillium, Fusarium, Trichoderma and a number of phycomycetes affect the seed of all forest species. The moisture content of the product enhances the development of these fungi. Also, temperature, storage time and degree of fungal contamination prior to storage, insect and mite activity facilitate fungi dissemination [7] . Pacin et al. [11] identified Aspergillus and Fusarium species in stored grains and they were found mycotoxic at different concentrations.
A very important tool as a complementary technology to boost maize production is the control of maize diseases. Breeding resistant varieties of maize, chemical treatment and biological control are some approaches that have been used over the decades to control maize diseases [12] . Synthetic fungicides have greatly contributed to management of losses due to aflatoxin-producing fungi in stored products. However, long term problems have resulted due to indiscriminate application of synthetic fungicides and ingestion of hexachlorobenzene (HCB) leading to toxic porphyria or poisoning in humans [13] . Synthetic fungicides such as captan, captafol and folpet are widely used to protect maize seeds but are responsible for symptoms such as skin irritation, dermal sensitization and several respiratory problems [14] .
Neem is native of India, Pakistan, Thailand, Burma and it belongs to the family Meliaceae. The amazing tree has been a help to human race since 4500 years ago [15] . One of the immediately perceivable impact of this antifungal, antibacterial and perhaps even antiviral king of the arboretums on the human body is its guaranteed ability to heal or cure many, if not all, skin diseases or epidermal problems ranging from dandruff, acne, psoriasis, ringworm, athlete's foot, warts, chicken pox, small pox and malaria [16] .
Garcinia kolakola Heckel (Ciusiaceae), commonly known as bitter kola (English), orogbo (Yoruba) and akinu (Igbo) is a widespread tree of evergreen forest valued in Nigeria for its medicinal nuts which has led to its exploitation in the natural forests in recent times [17] . It is chewed extensively in Southern Nigeria as a masticatory to cause nervous alertness and has been proven to exhibit pharmacological uses in treating coughs, and throat infections [17] . G. kola exhibits purgative, antiparasitic, anti- inflammatory, anti-bacterial and anti-viral properties [18] . In addition, G. kola enjoys a folk reputation in the management of sickle cell disease (SCD), as poison antidote [19] [20] and in the preservation of lipid food products prone to rancidity [18] .
Synthetic fungicides cannot be safely applied to maize grains for reasons of pesticide toxicity, though they may be effective and efficient for the control of seed-borne fungi [21] [22] . Therefore, there is a need to research for an alternative, eco-friendly and cost effective approaches for storing grains/cereals without toxicity problems. Plant extracts of many higher plants have been reported to exhibit antibacterial, antifungal and insecticidal properties [13] - [28] . One of the best alternatives is the plant metabolites and plant-based pesticides as they are known to have minimal environmental impact and danger to consumers in contrast to the synthetic pesticides [29] . Therefore, the present study was focused on the antifungal effect of neem and G. kola.
2. Materials and Methods
2.1. Sources of Plant Materials
1) Maize (Zea mays L.) seeds: Relatively healthy maize seeds (not freshly harvested) were obtained from Awka and stored in sterile paper bags.
2) Neem (Azadirachta indica A. Juss): Neem seeds were obtained from neem trees located within Nnamdi Azikiwe University, Main Campus Awka.
3) Bitter kola (Garcinia kola H.) seeds: Bitter kola seeds were procured from Eke-Awka market, Awka, Anambra State.
All plants were identified and authenticated by the curator attached to the Herbarium at the Department of Botany, Nnamdi Azikiwe University, Awka.
2.2. Media Preparation and Isolation
Sabouraud Dextrose Agar (SDA) was used for the isolation of fungi associated with maize seeds and for the sub-culture, growth and maintenance of the fungal isolates. SDA was prepared according to the manufacturer’s prescription and autoclaved at 121˚C for 15 minutes [30] [31] . All measurements were done using the weighing balance.
Direct isolation method was employed. Twenty relatively healthy maize seeds were surface-sterilized with 10% sodium hypochlorite (NaOCl) solution for 10 minutes and rinsed in three changes of sterile distilled water and left to dry on sterile filter papers [32] . Five maize seeds were inoculated per Petri dish and incubated at 28˚C ± 2˚C for 5 days. The Petri dishes were sealed with paraffin to prevent contamination.
2.3. Plant Extract
Fresh and healthy seeds of Azadirachta indica A. Juss were de-pulped and washed with sterile distilled water while Garcinia kola H. seeds were peeled and sliced with a sterile blade. These plant samples were air-dried at room temperature (25˚C) and turned into powder using a manual grinder. Solvents used for extraction were ethanol and methanol. Cold solvent extraction technique was employed [33] . Five hundred grams (500 g) of each plant sample was dissolved in 1 litre of ethanol or methanol separately and left to stand for 48 hours at 25˚C. The mixture was filtered using Whatman No 1 filter paper and the filtrate was concentrated with a rotary evaporator at 75˚C (for ethanol) and 65˚C (for methanol). The crude extracts were collected in sterile sample bottles and stored at 4˚C.
2.4. Antifungal Assay
Different concentrations of the test extracts were prepared by dissolving the extracts in the solvent; 25 mg/10ml, 50 mg/10ml, 75 mg/10ml and 100 mg/10ml. One millilitre (1 ml) of each plant extract solution was dispensed per Petri dish and 9 ml of molten SDA was added to each of the extracts to correspond to 2.5%, 5.0%, 7.5% and 10.0% extract concentration. Petri dishes were placed on a shaker for 10 minutes for even dispersion. Five millimetres (5 mm) disc of 5-day old pure culture of each test fungus was placed at the centre of the Petri dishes and incubated at 28˚C ± 2˚C. The negative control set up consists of un-amended agar plates while the positive control consists of the solvents mixed with the agar. The experimental design was a Completely Randomized Design (CRD) with 3 replicates. All plates were sealed with paraffin and radial growth was measured for five days. Colony diameter was taken as the mean along two directions on two perpendicular lines drawn on the reverse side of the plates.
Percentage inhibition was calculated according to Whipps [34] .
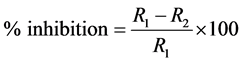
where R1 is the furthest radial distance of pathogen in control plates and R2 is the furthest radial distance of pathogen in extract-incorporated agar plates.
The inhibition percentage was determined as a guide in selecting the minimum inhibition concentration (MIC) that will be effective in controlling the fungi for their inhibitory effects using the scale of Sangoyomi [35] .
≤0% no inhibition
>0% - 20% slightly inhibition
>20% - 50% moderate inhibition
>50% - 100% inhibition (effective)
100% high inhibition
2.5. Statistical Analysis
Data were analyzed using ANOVA via SPSS. The means were separated using LSD at p ≤ 0.05.
3. Results
3.1. Percentage Occurrence of Fungi from Maize Seeds
Fungal isolates associated with maize observed in this study include: Aspergillus flavus, A. parasiticus, A. niger and Fusarium oxysporum. The most frequently occurring fungus was A. flavus (88.8%) while F. oxysporum was the lowest with 33.3%.
Aspergillus flavus, A. parasiticus, A. niger and Fusarium oxysporum were isolated from the maize sample. Pure cultures of A. flavus formed yellow colonies on SDA and the morphological features revealed that A. flavus forms mycelia that are white with spreading yellow colonies. The microscopic characteristics of the fungus include long non-septate cells borne on the hyphae.
3.2. Effect of Test Plant Extracts against Test Organisms
Result of methanolic extract of the test plant against A. flavus revealed that at 2.5% concentration, the percentage inhibition of A. flavus was highest in G. kola (45.6) and lowest in neem seed (35.1). At 5% concentration, the percentage inhibition of A. flavus was highest in G. kola (68.4) and lowest in neem seeds (54.7). At 7.5% concentration, the percentage inhibition of A. flavus was highest in G. kola (73.7) and lowest in combination of (G. kola and neem) seeds (61.1). At 10.0% concentration, the percentage inhibition of A. flavus was highest in G. kola (77.5) and lowest in neem seeds (64.9). There is a significant difference in the percentage inhibition of A. flavus between methanol extract of G. kola, neem and combination of (G. kola and neem) seeds at 10% concentration (p < 0.05) (Figure 1).
Ethanolic extract of the test plant against A. flavus indicated that at 2.5% concentration, the percentage inhibition of A. flavus was highest in combination of (G. kola and neem) seeds (58.9) and lowest in G. kola (31.4). At 5% concentration, the percentage inhibition of A. flavus was highest in combination of (G. kola and neem) seeds (69.6) and lowest in G. kola (38.2). At 7.5% concentration, the percentage inhibition of Aspergillus flavus was highest in combination of (G. kola and neem) seeds (73.4) and lowest in G. kola (45.6). At 10.0% concentration, the percentage inhibition of A. flavus was highest in combination of (G. kola and neem) seeds (80.4) and lowest in G. kola (53.6). Here, there is a significant difference in the percentage inhibition of A. flavus between ethanol extract of G. kola, neem and combination of (G. kola and neem) seeds at 2.5%, 5.0% and 10% concentration (p < 0.05) (Figure 2).
Methanolic extract of test plants against A. parasiticus indicated that at 2.5% concentration, the percentage inhibition of A. parasiticus was highest in G. kola (43.8) and lowest in mixture of G. kola and neem seeds (38.1). At 5% concentration, the percentage inhibition of A. parasiticus was highest in G. kola (46.2) and lowest in neem seeds (30.5). At 7.5% concentration, the percentage inhibition of A. parasiticus was highest in the combination of (G. kola and neem) seeds (55.2) and lowest in neem seeds (38.0). At 10.0% concentration, the percentage inhibition of A. parasiticus was highest in combination of (G. kola and neem) and neem seeds (54.8) and lowest in G. kola (54.3). There is a significant difference in the percentage inhibition of A. parasiticus between methanol extract of G. kola, neem and combination of (G. kola and neem) seeds at 7.5% and 10% concentration (p < 0.05) (Figure 3).
Result of ethanolic extract of the test plant showed that at 2.5% concentration, the percentage inhibition of A. parasiticus was highest in G. kola (54.6) and lowest in the combination of (G. kola and neem) seeds (26.6). At 5%
![]()
Figure 1. Percentage inhibition of Aspergillus flavus using methanol extract of G. kola, neem and combination of (G. kola and neem) seeds.
![]()
Figure 2. Percentage inhibition of Aspergillus flavus using ethanol extract of G. kola, neem and combination of (G. kola and neem) seeds.
![]()
Figure 3. Percentage inhibition of Aspergillus parasiticus using methanol extract of G. kola, neem, and combination of (G. kola and neem) seeds.
concentration, the percentage inhibition of A. parasiticus was highest in G. kola (65.5) and lowest in combination of (G. kola and neem) seeds (44.2). At 7.5% concentration, the percentage inhibition of A. parasiticus was highest in G. kola (76.7) and lowest in combination of (G. kola and neem) seeds (39.3). At 10.0% concentration, the percentage inhibition of A. parasiticus was highest in G. kola (79.5) and lowest in combination of (G. kola and neem) seeds (48.1). There is a significant difference in the percentage inhibition of A. parasiticus between ethanol extract of G. kola, neem and combination of (G. kola and neem) seeds (p < 0.05) (Figure 4).
4. Discussion
Four fungal isolates were identified in this study, of which three were Aspergillus species with A. flavus having the highest frequency of 88.88%. According to Yan-ni et al. [36] , Aspergillus species are found to be associated to disease in maize. The result of high frequency of A. flavus in maize agrees with the report by Bankole et al. [37] and Wagacha and Muthomi [38] . Methanolic and ethanolic extracts of A. indica and G. kola seeds at four concentrations (2.5%, 5.0%, 7.5% and 10.0%) were observed in this study to inhibit the growth of A. flavus and A. parasiticus. Kaur and Kaur [39] had reported that natural products of higher plants give a new source of antimicrobial agents with possible novel mechanism of action. This is supported by Kiran et al. [40] , who screened seven medicinal plants for anti-fungal activity against seed-borne fungi of maize seeds.
In this study, the ability of the test plants extracts (individually and in combination) to inhibit the growth of A. flavus and A. parasiticus varied. Results showed that the test plants extracts were effective for reducing mycelial growth of test organism. Methanolic extract of G. kola, neem and combination of (G. kola and neem) against A. flavus showed antifungal activity in the inhibition which was highest in G. kola at 10% concentration. With ethanolic extracts of G. kola, neem and combination of (G. kola and neem) against A. flavus, the inhibitory activity was highest in the combination of G. kola and neem at 10% concentration. Methanolic extracts G. kola, neem and combination of (G. kola and neem) against A. parasiticus showed that inhibition was highest in the combination of G. kola and neem at 7.5% concentration. In the ethanolic extracts of G. kola, neem and combination of (G. kola and neem) against A. parasiticus, inhibition was highest in G. kola at 10% concentration.
![]()
Figure 4. Percentage inhibition of Aspergillus parasiticus using ethanol extract of G. kola, neem, and combination of (G. kola and neem) seeds.
In all, it can be seen from the study that G. kola and neem seeds produced good result (with slight variations) in inhibiting the growth of A. flavus and A. parasiticus and the solvents (methanol and ethanol) used were effective in extracting the active ingredients from the test plants. The extracts from these plants showed the ability to suppress growth of A. flavus and A. parasiticus. It is possible that these bioactive compounds may be responsible for the antifungal activity and hence the efficacy against A. flavus and A. parasiticus. This result confirms the report given by Joseph et al. [41] , that extract from Garcinia have rich source of phenolic acids that inhibited aflatoxin producing fungi and that neem seed powder is a very good biological fungicide [9] .
5. Conclusion
Synthetic fungicides have been used for the preservation of stored grains, but due to long effects on human health, natural plant extracts may provide a better alternative in other to produce safe food. Much attention has been given to the use of medicinal plant to control plant diseases. This has led to the use of plant as excellent bio-fungicide. The two plant extracts used in this study contained effective phytochemical compounds that could be used as inhibitors of fungal growth in maize grains and also in other plant grains or cereals. The bioactive substance in these plants indicates that these plants are potential for managing A. flavus and A. parasiticus production in maize grains. In adapting the methods used in this study to control A. flavus and A. parasiticus, consideration should be given to the use of different concentrations of the crude plant extracts, and the test plant extracts as this had variations in inhibiting the growth of the organisms. This study also recommends evaluation of other medicinal plants as a bio-fungicide against A. flavus and A. parasiticus.
NOTES
![]()
*Corresponding author.