Nutrient Fluxes and Sediments Composition in El Mex Bay and Surround Drains, Alexandria, Egypt ()
1. Introduction
During the last century, the Egyptian coastal areas along the Mediterranean sea were strongly impacted by the development of anthropogenic activities on their shores and the subsequent inputs of inorganic including nutrients and organic pollutants [1] -[7] . Although these releases were significantly reduced during recent decades because of regulations, contaminants accumulated in the sediments over time [8] - [19] . These sediments now constitute a potential source of contaminants for the water column that could alter the water quality and threaten aquatic organisms [20] - [30] . Understanding the processes controlling the dynamics of contaminants in the sediment, improving our knowledge of the environmental risk induced by contaminated sediments and appropriately orientating the action of politics and managers, is thus a challenge for the next decades [31] .
Nutrient fluxes at the sediment-water interface can indeed influence or regulate the nutrient composition of the water column since the sediment can behave as a sink or as a source of inorganic nitrogen, phosphorus and silicate through different biogeochemical processes [32] . Processes involved in nutrient transfer are reversible, quick and differ with season and sediment types. Since many pollutants originally introduced into the water column have affinities for sediment particles, the pore water is expected to be more polluted than bottom water [1] . Nutrient salts had been introduced to El Mex Bay mainly through the surrounded drains [33] .
Sediments may originate from a number of sources. The proportions of sediments from different sources at any particular location will depend on a variety of hydrological and geological factors, e.g. circulation patterns, tidal movement, weathering conditions and source rocks. It was pointed out that the two extreme sources of sediments i.e. landward and seaward, together with intermediate sources, such as river mouths slope impose severe limitations on the geochemical interpretation of sedimentary processes [34] . The most important sedimentary and chemical interactions may be subdivided into two aspects; 1) the modification of sedimentary detritus during its transport to the seas; and 2) the modification of sediments after deposition (digenetic changes). IR, X ray and DTA are used to study sediment compositions.
2. Materials and Methods
2.1. Study Area
El-Mex Bay is part of the Alexandria coast on the Mediterranean Sea. It is adjacent to the center of Alexandria that is populated with about six million inhabitants and is considered as one of the main fishing sources in Egypt. It extends for about 15 km between El-agamy head land in the west and the western harbor to the east and from the coast to a depth line of about 15 km. the bay has a mean depth of about 10 m and surface area of about 19.4 km2 (Figure 1). It is a highly polluted area, the major types of pollution sources are domestic sewage, industrial waste water, and agricultural run-off, through lake out lets, and river discharged and oil pollution. El-Mex Bay receives mixed agricultural run-off from lake Mariut through El-max pumping station and El-umum drain, industrial water from chloro-alkali plant, tanneries and slaughterhouse, also, air borne particles from the fumes of adjacent industrial plants including a cement factory [35] - [42] .
![]()
Figure 1. El-Mex Bay and surround drains showing sampling stations.
2.2. Sampling
2.2.1. Bottom Water
Bottom water samples were collected using a five liters Nisken’s plastic bottle provided with a thermometer.
2.2.2. Grab Surface Sediments
Surficial sediments were collected in April 2010 from seven stations, distributed at El-Mex Bay of Alexandria, and four stations in the drains (El-Umum, El-Noubaria, El-Qalah and Mariout Lake) as shown in Figure 1 using Ekman grab sampler.
2.3. Methods
2.3.1. Bottom Water
Nutrient salts were spectrophotometrically determined using a double beam spectrophotometer (UV VIS- SPEKOL® 1300/1500 single beam), according to the methods described by Strickland and Parsons [43] .
2.3.2. Interstitial Waters
a) Extractions of Interstitial Waters from Sediments
The choice of the techniques used for extraction the interstitial water from sediments is usually governed by the nature of sediments and available facilities as follows; 1―In case of silty sediment fractions, the squeezer technique was used [44] . The squeezer was lined with Teflon as recommended by Patterson and Settle [45] ; centrifugation for muddy sediments occurred at 10.000 rpm for about 20 minutes [46] ; and 2―by filtration on a glass fiber filter, in case of sand sediment fractions.
b) Nutrient Salts Analysis
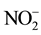 ,
, 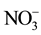 ,
, 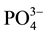 and
and 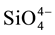 were measured in small volumes of the interstitial waters (5 - 10 ml). Nutrient salts were spectrophotometrically determined using a double beam spectrophotometer (UV VIS-SPEKOL® 1300/1500 single beam) according to Strickland and Parsons [43] .
were measured in small volumes of the interstitial waters (5 - 10 ml). Nutrient salts were spectrophotometrically determined using a double beam spectrophotometer (UV VIS-SPEKOL® 1300/1500 single beam) according to Strickland and Parsons [43] .
2.3.3. Sediments
a) Preparation of Samples
After extraction of the interstitial waters, sediment samples were subjected to air dryng, by spreading them on clean plastic sheets. All these were made inside a clean cabinet. The quartering was made using the familiar cone and quarter technique. Air dried samples were placed inside an electric oven for overnight at 70˚C. One half of each dry sediment sample was lightly hand ground in an a agate mortar, sieved through a screen of 0.2 mm mesh size and kept in clean and well stopper polyethylene vials to be ready for geochemical analysis. The remainder of each dry sample was used for the mechanical or grain size analysis.
b) Grain-size and Granulemetric Analysis
The sediment samples were subjected to grain size analysis according to the method described by Folk [47] . The phi unit is equivalent to the −log 2x.where x is the grain size in millimeter. 25 g of the dry sediment samples were taken for mechanical analysis, which was carried out using a standard set of sieves in a Ro-Top shaker for 20 min, the sieves were arranged from top to bottom in a one phi order as follows: −2, −1, 0, 1, 2, 3 and 4 phi, which correspon 4, 2, 1, 0.5, 0.25, 0.125 an 0.063 mm respectively. The collected sieve fractions were accurately weighted. The samples containing an appreciable amount of mud (more than 10%) were subjected to pipette analysis as described by Krumbein and Pettijhon [48] . Each fraction of sucking pipette was dried and weighted to the nearest 0.0001 g.
c) Infrared Spectra (IR) Analysis
Sediment samples were analyzed by Infrared Perkin-Elmer R79521, Ratio Recording FT-IR System Spectrophotometer (USA) that was available from central lab unit, National Institute of Oceanography and Fisheries (NIOF), Alexandria, Egypt.
d) X-ray analysis
The data were obtained using (Pentater Link Oxford, Link ISIS) and JEOL JSM-5300 Scanning electron Microscope. Specttra taken in the range of 1 - 8 Kev. The instrument is available in faculty of science Alexandria University.
e) Thermal analysis
2.4. Fluxes Calculations
Due to the ever-increasing loads of nitrogen and phosphorus from aquaculture, lots of dissolved or granular nitrogen and phosphorus accumulate on the surface of the sediments by flocculation, adsorption and sedimentation, resulting in growing contents of nitrogen and phosphorus in the overlying waters. This indicates that in El Mex Bay, a large amount of nitrogen and phosphorus from the sediments is released into the overlying water [33] [37] - [39] . It is obviously clear that there are concentration gradients of nitrogen and phosphorus in pore water along the sediment depth. The diffusion fluxes of nitrate, nitrite and dissolved reactive phosphate (DRP) and silicate across sediment-water interface can be computed by Fick’s first law. Fluxes of ammonia, nitrite, nitrate, phosphate and silicate between sediments and the overlying water were calculated according to Fick’s first law of diffusion as follows:
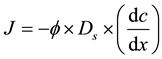
where J is the flux (μmol・m−2・d−1), 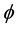 is the mean porosity of surface sediment (mlpore water/cm3sediment; dimensionless) = 0.7 for El Mex Bay (unpublished data), Ds is the diffusion coefficient (m2・d−1), and dc/dz is the concentration gradient in the overlying bottom waters (μmol・m−4).
is the mean porosity of surface sediment (mlpore water/cm3sediment; dimensionless) = 0.7 for El Mex Bay (unpublished data), Ds is the diffusion coefficient (m2・d−1), and dc/dz is the concentration gradient in the overlying bottom waters (μmol・m−4).
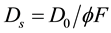 [49]
[49]
where D0 is the diffusive coefficient at infinite dilution, and the values for NO2-N, NO3-N, PO4-P, SiO4-Si were 18.3, 17.2, 9.25, 7.07 × 10−6 (cm2/sec) at 21˚C [50] and F is the sediments resistivity [51] .
Krom and Berne [49] , gives an empirical relationship between F and ![]() according to:
according to:
![]()
For![]() , m = 2 is a better fit to the data. These data imply that m is not constant over the entire range of porosities possible in a particular sediment type undergoing natural compaction.
, m = 2 is a better fit to the data. These data imply that m is not constant over the entire range of porosities possible in a particular sediment type undergoing natural compaction.
The diffusion flux (Ji) for phosohorus is estimated by Fick’s law according to:
![]()
Therefore: ![]()
![]()
where ![]()
![]()
![]()
![]()
![]()
Sakamaki et al., [52] reported that the sediment water exchange fluxes of ![]() are controlled by both benthic microalgal uptake and the release from sediments that is largely affected by the overlying water quality. The authors added that at high concentration in low tide the benthic microalgal actively absorbs dissolved inorganic nitrogen, especially
are controlled by both benthic microalgal uptake and the release from sediments that is largely affected by the overlying water quality. The authors added that at high concentration in low tide the benthic microalgal actively absorbs dissolved inorganic nitrogen, especially ![]() that is much higher than
that is much higher than ![]() and high transfer of
and high transfer of ![]() from water to sediments occurs. In this case, the activity of algae is also considerably affected by the high concentration of
from water to sediments occurs. In this case, the activity of algae is also considerably affected by the high concentration of ![]() and the uptake fluxes largely change during the day time. However, at low concentration in high tide, a high release from sediments to water occurs and predominates in the sediment-water fluxes.
and the uptake fluxes largely change during the day time. However, at low concentration in high tide, a high release from sediments to water occurs and predominates in the sediment-water fluxes.
3. Results and Discussion
3.1. Nutrient Exchange between Pore and Overlying Water
Measurements of dissolved inorganic nutrients (![]() ,
, ![]() ,
, ![]() and
and![]() ) in PW and BW were performed in El Mex Bay and surround drains during Spring 2010, and illustrated in Table 1. The nutrient salts concentrations in drains are much higher than El Mex Bay stations because of agriculture and domestic wastes.
) in PW and BW were performed in El Mex Bay and surround drains during Spring 2010, and illustrated in Table 1. The nutrient salts concentrations in drains are much higher than El Mex Bay stations because of agriculture and domestic wastes.
When nutrients from outer sources are discharged into water bodies, a great deal of nitrogen and phosphorus accumulates in sediments and their concentrations may be up to 50 to 500 times that in the overlying water [53] . The results showed significantly higher nutrient concentrations in the PW than their corresponding BW as shown in Table 1. The average concentrations in PW were about 44.8, 37.6, 113.45 and 15.76 times higher than the average values reported in the BW for![]() ,
, ![]() ,
, ![]() and
and ![]() respectively in El Mex Bay. The large increase in nutrient loading has led to the impairment of many water bodies globally [54] . This included the eutrophication of water bodies that can lead to dissolved-oxygen depletion, species shifts, and fish kills [55] . Nutrient concentrations in the PWin the present study had higher average values than reported by Nessim et al., [56] in Eastern Harbour (2.8. 14.2 and 25 for
respectively in El Mex Bay. The large increase in nutrient loading has led to the impairment of many water bodies globally [54] . This included the eutrophication of water bodies that can lead to dissolved-oxygen depletion, species shifts, and fish kills [55] . Nutrient concentrations in the PWin the present study had higher average values than reported by Nessim et al., [56] in Eastern Harbour (2.8. 14.2 and 25 for![]() ,
, ![]() and
and ![]() µML−1, respectively).
µML−1, respectively).
Nutrient diffusive fluxes calculated in the present study were illustrated in Table 2 that had averages of −7.24, −1.36, −7.86 and −1.33 in the upword diriction, however, in the drains were −34.39, −32.28, −53.20 and −117.6 mg・m−2・day−1 for![]() ,
, ![]() ,
, ![]() and
and![]() , respectively. Abu El Khair et al., [57] reported difussive fluxes of −0.053, −0.445 and −1.77 mg・m−2・day−1 for
, respectively. Abu El Khair et al., [57] reported difussive fluxes of −0.053, −0.445 and −1.77 mg・m−2・day−1 for![]() ,
, ![]() and
and![]() , respectively in Abu Qir Bay. Additionaly, Farragala [58] reported nutrients diffusive fluxes of −0.01, +0.007 and −0.143 mg・m−2・day−1 for
, respectively in Abu Qir Bay. Additionaly, Farragala [58] reported nutrients diffusive fluxes of −0.01, +0.007 and −0.143 mg・m−2・day−1 for
![]()
Table 1. Nutrient concentrations in BW and PW (µML−1) of El-Max bay and surrounded drains during spring 2010.
8―El-Umum Drain, 9―El-Qalaa Drain 10―El-Noubaria Drain and 11―Mariut Lake.
![]()
Table 2. Fluxes of nitrite, nitrate, phosphate and silicate (mg・m−2・day−1) from sediments in El-Mex bay and surround drains during spring 2010.
8―El-Umum Drain, 9―El-Qalaa Drain 10―El-Noubaria Drain and 11―Marriut Lake. D0; is the diffusive coefficient at infinite dilution at 21˚C cited from Zhang et al. [50] .
![]() ,
, ![]() and
and![]() , respectively. Comparing the results of the present study with that reported by Abu Khair et al., [57] and Faragalla [58] revealed that nutrient upword diffusive fluxes in El Mex Bay had significant higher values that recorded in Abu Qir Bay and Eastern Harbour.
, respectively. Comparing the results of the present study with that reported by Abu Khair et al., [57] and Faragalla [58] revealed that nutrient upword diffusive fluxes in El Mex Bay had significant higher values that recorded in Abu Qir Bay and Eastern Harbour.
![]() and
and ![]() diffused from pore water with high values refered to that immobilization rate (Nitrification) of these ions is greater than consumption rate (denitrification). This reflects the dominant of oxidation reaction in pore water in the study area. Dissolved Oxygen in the bottom water in the study area during Spring was 6.61 mg/L [33] , however DO was low most of the year in the Eastern Harbour 3.2 - 5.7 mg/L [59] .
diffused from pore water with high values refered to that immobilization rate (Nitrification) of these ions is greater than consumption rate (denitrification). This reflects the dominant of oxidation reaction in pore water in the study area. Dissolved Oxygen in the bottom water in the study area during Spring was 6.61 mg/L [33] , however DO was low most of the year in the Eastern Harbour 3.2 - 5.7 mg/L [59] .
During Spring, when the surface cools, a point is reached at which the temperature of the surface and bottom are equal. The disappearance of thermal stratification cause the entire body of water to behave as a hydrological unit, and the resultant mixing is known as overturn. During the overturn, the chemical and physical characteristics of any body of water becomes much more uniform, and a number of chemical, physical, and biological changes may result. Biological activity may increase from the mixing of nutrients. Higher and negative flux values (upword flow from sediment to over laying water) of all nutrients could be attributed to higher sediment organic matter content and high biological activity. Delange [60] suggested at least three scenarios for ultimate source of phosphorus; Organic-P cracking, the release of phosphorus from phosphor-lipids and other high energy phosphorus compounds from plankton debris by microbial action at the sediment-water interface; dissolution of fish debris; and the release of phosphorus sorbed onto iron oxyhydroxides, i.e. FeOOH sorbs phosphorus from bottom water and from upward diffusion PW phosphate and incorporate below the oxic surface sediments by burial or mixing, are reduced to Fe2+, releasing the sorbed phosphate. Noel [61] reported the sediment compartment plays a role in the water column phosphate contents and should be considered as a buffer able to both store and release phosphate according to the conditions. However through exchanges at the sediment-water interface and migration of the phosphate, The exchange process that in spring, biological activity was more stressed and a steady equilibrium cannot be reached.
3.2. Grain size (Texture) Analysis
Mean grain size and the median diameter may reflect the general characteristics of granule metric composition of sediment. While the values of skewness and kurtosis reflects the uniformity of distribution of sediment composition. The distribution of sediment composition depends on the equilibrium between gravity of sediments and water forces. In the present study, the results of the grain size analysis of the grab sediments of the study area, (Table 3). Most of the sediments of El-Mex Bay are rich in sand fraction. The respective ranges of silt are from 0.00% at station 3 to 91.20% at station 5. For clay fraction, the respective ranges were 0.00% at station 6, 7 (front of western harbor and Far 1200 m from Station 4) to 9.12% at station 1 (El-Dekhila Head). The respective ranges of sand are 0.00% at station 1 (El-Dekhila Head) to 100% at stations 3, 4, 6 and 7 (Petrochemicals company, in front of El-Umum drain and western harbor and far 1200 m from EL-Umum drain).
El-Mex sediments contained appreciable amounts of tubeworm skeletons of some calcareous organisms, bivalve shell fragments (placipoda, plyciopoda and gastropod) and gravel. The texture of sediments was mainly sandy, with some muddy sediments in stations 1, 2 and 5 (Table 3). These sediments are exposed more to the sea and current actions leading to such good sorting and dominance of the coarser sandy fraction. The dominance is sandy mud sediments in the most stations. Most of the sediments of El-Mex drains are rich in silt fraction. The respective ranges of sand are from 2.36% at station 11 (El-Qalaa drain) to 39.30% at station 9 (El-Umum drain). Clay fraction ranged from15.74% at station 9 (El-Umum drain) to 37.72% at station 8 (El-Noubaria drain). The ranges of slit are 44.97% at station 9 (El-Umum drain) to 77.06% at station 10 (Mariut Lake).
3.3. Infrared Spectra (IR)
IR curves are normally used in mineralogy for qualitative analysis and identification of different minerals, even complex mixtures. It is based on positions and shapes of absorption bands [62] . Spectra of all sediment samples are very nearly similar indicating that the constituents are almost the same. The main features of the sediment absorption spectra (Figure 2) showed a broad band within the range 3100 - 3600 cm−1 assigned for O-H stretching vibration of H2O which readily lost upon heating [62] [63] . These regions are mainly composed of calcareous sediment containing amorphous silica SiO2nH2 O) according to Degens [64] .
A weak band within the range of 2300 - 2400 cm−1 is characteristic for absorption of carbonate minerals (calcite and magnesium calcite) according to Smolander et al., [62] . The samples gave weak bending vibration bands within the range of 1700 - 1800 cm−1, reflecting the deformation of water molecules in clay minerals [63] [65] . Furthermore, the spectra of all samples showed strong broad feature at 1400 - 1500 cm−1 characteristic for carbonate radical [62] . The in plane bending vibration band within the range 1030 - 1090 cm−1 for O-H in the sediment samples appears in all regions. A peak can be seen, especially at El-Mex and El-Qalaa around 2940
![]()
Table 3. Grain size analysis of El-Mex Bay and drains during spring 2010.
8―El-Umum Drain, 9―El-Qalaa Drain, 10―El-Noubaria Drain, and 11―Mariut Lake.
![]()
Figure 2. Infrared of sediment samples (a) Mariut Lake, (b) El-Mex, (c) El-Qalaa.
cm−1 could be attributed to the asymmetrical C-H stretching of methyl (-CH2) groups being characteristic of aliphatic hydrocarbon [66] . The bands within the range of 780 - 800 cm−1 represent the bending in plane vibration band of OH groups for δMgAlOH [67] . Carbonate minerals in all sediments are identified by the wave number of M-CO3 stretching band within the range of 650 - 700 cm−1. The symmetric Si-O-Si stretching appeared as weak bands at 460 - 500 cm−1 for Mariut and El Qalla drains sediment samples [67] .
3.4. X-Ray Analysis for Mineral Analysis of Sediments
Figures 3-5 and Tables 4-6 showed that the sediments comprise two main minerals: silicate and carbonate that appear in some sediments profile as Mg-calcite as a result of Mg substitution [68] . Silicate minerals could be differentiated into quartz and feldspar. For El-Max Bay, the sediment sample mainly comprises calcium 91%, silicate 4.2%, 1.7% sulfides and 1.4% Manganese. In El-Qalaa, the sediment sample comprises of silicate 70.7%, Manganese 6.7% and 6.3% Calicum. In mariut Lake, the sediment sample mainly comprises of Silicate 46.9%, calcium 18.1% and 13.4% Manganese. The X-Ray analysis results peformed for the present study reflecting predominantly of Calcium, Silicate and Manganese minerals.
3.5. Differential Thermal Analysis (DTA)
Differential thermal analysis is a thermoanalytic technique. In DTA, the material under study and an inert reference are made to undergo identical thermal cycles, while recording any temperature difference between sample and reference [69] . The DTA is illustrated in Figure 6 for station 4 of El-Mex Bay, El-Qalaa and Mariut Lake Sediment samples. El Mex Bay sample showed endothermic decomposition to CaO and CO2 at 700˚C - 900˚C [70] (Sestak, 2005). The small peaks observed for El-Qalaa drain and Mariout lake, samples indicated the presence of quartz (SiO2) because of the α to β quartz inversion. The calculated order values from the peak asymmetry method [71] are (n = 0.46, 1.45 and 1.45) for El-Mex Bay, El-Qalaa and Mariout Lake sediment samples. The order of reaction indicates that the reactions are not of simple manner but the reactions proceeded in complicated mechanisms. The ?ΔS# values support that the sediments are more ordered and such structures are hardly subjected to decomposition. The small collision factor values (Z), support the view of the extra stability of these sediments [72] [73] .
DTA figures gave steps due to dehydration, rearrangement and decarbonation. Table 7 illustrated that the order of the reactions is ~1. The −ΔS values support that the sediments are more ordered. So, such structures are hardly for decomposition. This is well verified by −ΔH values. The −ΔS values are nearly of the same magnitude, revealing that the composition of the sediment samples is the same independent of the region of the studied area. The small collision factor values, Z, support the view of the extra stability of these sediments [18] [74] [75] .
![]()
Figure 3. X-Ray analysis peaks for a) El-Mex Bay.
![]()
Figure 4. X-Ray analysis peaks for b) Mariut lake.
![]()
Figure 5. X-Ray analysis peaks for c) El Qalaa drain.
![]()
Table 4. X ray for El Mex Bay station 4.
![]()
Table 5. X ray analysis for Mariut lake.
![]()
Table 6. X ray analysis for El Qalaa drain.
![]()
![]()
![]()
Figure 6. The DTA peaks for station 4 of El-Mex Bay, El-Qalaa and mariut lake sediment.
![]()
Table 7. Thermodynamic parameters (DTA) for sediment samples.
4. Conclusion
The inorganic nutrient concentrations in the BW column of El Mex Bay and its surround drains indicate that this area suffers from acute eutrophication, resulting from a great amount of anthropogenic nutrients entering the sea through numerous land-based sources. Continuous burial and decomposition of organic matter in the topmost layer of the sediment is the main reason for the high nutrient concentrations in the PW compared to those in the OBW. The variations in concentrations between sites can be mainly attributed to variations in supply and input of reactive organic matter. Nutrient diffusive fluxes calculated in the present study during spring, 2010 from sediments had averages of −7.24, −1.36, −7.86 and −1.33, however in the drains were −34.39, −32.28, −53.20 and −117.6 mg・m−2・day−1 for![]() ,
, ![]() ,
, ![]() and
and![]() , respectively. Based on IR X-ray diffraction and DTA sediment samples were mainly composed of calcite, Mg-calcite and silicate.DTA curves are used to evaluate and discuss different kinetic parameters (n, Ea#, ∆G#, ∆H#, ∆S#, Z and Tm) support the view of the extra stability of these sediments.
, respectively. Based on IR X-ray diffraction and DTA sediment samples were mainly composed of calcite, Mg-calcite and silicate.DTA curves are used to evaluate and discuss different kinetic parameters (n, Ea#, ∆G#, ∆H#, ∆S#, Z and Tm) support the view of the extra stability of these sediments.
NOTES
*Corresponding author.