Formation of Supersaturated Solid Solutions Bi-Ag and Bi-Zn by Rapid Solidification Using Melt Spinning Technique ()
1. Introduction
Nowadays rapid solidification technology is used in manufacturing semiconductors. Rapid solidification can produce semiconductor alloys not normally obtained by conventional methods. The semiconductors prepared by rapid solidification exhibit considerable improvements over alloys made from conventional materials. The ther- moelectric materials fabricated by rapid solidification displayed a high figure of merit [1] . Formation of dendritic structure in thermoelectric alloys impairs its thermoelectric parameters [2] - [6] . Several authors studied the effect of alloying on the electronic and the electrical properties of Bi; they found that the addition of antimony changes the electrical behavior from semimetal to semiconductor at a certain range of concentration of Sb [7] - [9] . A series of rapidly solidified Bi-based semiconductor alloys are produced using melt spinning technique [10] [11] . They found that the semiconducting behavior depends on the type of the solute atoms added to Bi. The alloys contain solute atoms with odd valencies: for instance, Ag (+1), Al (+3) and Sb (+5) have semiconducting behavior by contrast; all alloys contain solute atoms with even valencies: for instance Zn (+2) and Sn (+4) have metallic behavior. According to the equilibrium Bi-Ag phase diagram, there is no solubility of Ag in Bi [12] . By rapid solidification using melt spinning technique, the solubility of Ag in Bi is extended to 2 at% [10] . There are significant discrepancies concerning the solubility limit of Zn in Bi prepared by conventional casting. Recent investigations indicate that the solubility of Zn in Bi is 0.3 wt% at 200˚C [13] [14] . In contrast some authors found that the solubility of Zn in Bi is 2 wt% [15] . Therefore, the aim of the present work is to study the effect of increasing Ag and Zn concentrations on the structure and electrical properties of Bi. Rapid solidification has been used in the present work to prevent rejection of extra solute atoms and thus prevent precipitation, from a solid solution.
2. Experimental Procedures
The materials used in the present work are Bi, Ag and Zn fragments the starting purity was better than 99.99%. Two alloys Bi-4.3 at% Ag (eutectic) and Bi-8.1 at% Zn (eutectic) were produced by a single copper roller (200 mm in diameter) melt-spinning technique. The process parameters such as the ejection temperature, and the linear speed of the wheel were fixed at 873 K and 30.4 ms−1 respectively. X-ray diffraction analysis (XRD) was carried out with a XPERT-PRO X-ray diffractometer, using Cu-Ka radiation (l = 1.5406 Å). Differential Scanning Calorimetry (DSC) was carried out in a Shimadzu (DSC-50) with heating rate 10 K・min−1. The temperature dependence of resistivity (TDR) was measured by four probe method using micro ohmmeter of type BS407. The BS407 uses a four terminal measurement system via high quality Kelvin Clip leads with sensitivity is 1 mΩ. The heating range starts from room temperature up to 530 K with heating rate about 5 K・min−1.
3. Results and Discussion
3.1. Structure
Figure 1(a) shows the X-ray diffraction pattern for as-quenched melt-spun Bi-4.7 at% Ag eutectic alloy. All the
![]()
Figure 1. X-ray diffraction pattern for as-quenched melt-spun: (a) Bi-4.7 at% Ag eutectic alloy; (b) Bi-8.1 at% Zn eutectic alloy.
peaks were identified for Bi rhombohedral structure. No precipitation of Ag was observed and the structure is a single phase Bi rich solid solution of Ag in Bi. According to the equilibrium phase diagram there no solubility of Ag in Bi. Therefore rapid solidification by melt spinning technique extended the solubility of Ag in Bi up to the eutectic composition. The structure of Bi is rhombohedral-hexagonal (S.G.: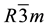 ) with lattice parameters [16] a = 4.745 Å, α = 57.23˚. Alternatively, the lattice parameters can be specified as the corresponding hexagonal vectors, with lengths a = 4.546 Å and c = 11.862 Å.
) with lattice parameters [16] a = 4.745 Å, α = 57.23˚. Alternatively, the lattice parameters can be specified as the corresponding hexagonal vectors, with lengths a = 4.546 Å and c = 11.862 Å.
By addition of Ag to Bi, the lattice parameters of Bi were increased (see Table 1). However the axial ratio c/a did not change. Also the volume of unit cell was increased. The intensity ratio from (110) to (012) planes was decreased from 29% to 14.64%. The particle size of Bi was calculated using Debye-Scherrer formula [17] to be 343.4 Å.
Figure 1(b) shows the X-ray diffraction pattern for as-quenched melt-spun Bi-8.1 at% Zn eutectic alloy. All the peaks were identified for Bi rhombohedral structure. No precipitation of Zn was observed and the structure is a single phase Bi rich solid solution of Zn in Bi. Therefore rapid solidification by melt spinning technique extended the solubility of Zn in Bi up to the eutectic composition. The lattice parameter a is increased and c is decreased and this led to the decrease in the axial ratio c/a (see Table 1). The volume of the unit cell v is increased from 212.298 to 212.608 Å3. The intensity ratio from (110) plane to (012) plane was decreased from 29% to 6.74%. The particle size was calculated to be 330.7 Å. The detail of XRD is shown in Table 1.
3.2. Thermal Analysis
Figure 2 shows the DSC curves for as-quenched melt-spun Bi-4.7 at% Ag and Bi-8.1 at% Zn alloys.
For Bi-4.7 at% Ag (Figure 2(a)) no phase transformation was observed before melting. The melting endotherm is a single sharp peak which characterizes the eutectic reaction. The melting temperature Tm was found to be 534.37 K. The enthalpy of fusion DHm was calculated to be 57.67 kJ∙kg−1. The same behavior was observed for Bi-8.1 at% Zn (Figure 2(b)).
![]()
Table 1. The detail of XRD results of rapidly solidified Bi- based alloys.
Bi*: Solid solution of Ag in Bi; Bi#: Solid solution of Zn in Bi.
![]()
Figure 2. DSC curves for as-quenched melt-spun: (a) Bi-4.7 at% Ag eutectic alloy; (b) Bi-8.1 at% Zn eutectic alloy.
No phase transformation was observed before melting and the melting endotherm is a single sharp peak which characterizes the eutectic reaction. The melting temperature Tm was found to be 527.45 K and the enthalpy of fusion DHm was found to be 61.26 kJ∙kg−1. The detail of DSC is shown in Table 2.
3.3. Electrical Properties
Figure 3(a) shows the TDR curves obtained for as-quenched melt-spun Bi-4.7 at% Ag alloy. The resistivity de- creases by increasing temperature i.e. it has a semiconducting behavior.
The resistivity at room temperature of pure Bi is 1.17 mWm and TCR is 4.6 × 10−3 K−1 [18] . Pure Bi was rapidly solidified by melt spinning technique and its resistivity was measured to be 2.09 mWm and TCR was found to be 3.05 × 10−3 K−1 [19] . Therefore rapid solidification increases the resistivity of Bi and decreases the TCR. The energy gap was calculated and was found to be 280 meV. The increase in Ag concentration increases the energy gap of as quenched melt spun semiconducting Bi-Ag alloy from 225 meV for Bi-1 at% Ag [10] to 280 meV for Bi-Ag eutectic alloy. The explanation of the semiconducting behavior of Bi alloys is given elsewhere [20] .
Figure 3(b) shows the TDR curves obtained for as-quenched melt-spun Bi-8.1 at% Zn alloy. The resistivity increases by increasing temperature i.e. it has a metallic behavior. The resistivity at room temperature was found to be 14.25 mWm and TCR was found to be 3.38 × 10−3 K−1. Both r and TCR were increased due to the addition of Zn (see Table 3).
![]()
Table 2. The detail of DSC results of rapidly solidified Bi-based alloys.
![]()
![]() (a) (b)
(a) (b)
Figure 3. TDR curves obtained for as-quenched melt-spun: (a) Bi-4.7 at% Ag eutectic alloy; (b) Bi-8.1 at% Zn eutectic alloy.
![]()
Table 3. The detail of TDR results of rapidly solidified Bi-based alloys.
CC: conventional casting; RS: rapid solidification.
4. Conclusions
From this work, the following conclusions may be obtained:
1) Rapid solidification using melt-spinning technique can produce semiconductor alloys, which cannot be normally obtained by conventional casting. The rapidly solidified Bi-Ag eutectic alloy was found to be a narrow gap semiconductor. Also by increasing Ag concentration, the energy gap increased from 225 meV for Bi-1 at% Ag [10] to 280 meV for Bi-Ag eutectic alloy.
2) From the XRD it is evident that the solid solubility of both Ag and Zn was extended to the eutectic concentration due to rapid solidification by melt spinning technique. Also the addition of both Ag and Zn to Bi increases the volume of the unit cell. However Ag extends the unit cell in both a and c directions keeping the axial ratio c/a without change, and in case of Zn the unit cell is increased in a direction and decreased in c direction leading to the decrease in the axial ratio c/a.
3) The increase in the Zn concentration in Bi did not lead to any semiconducting behavior. This indicates that the odd valency dopants are responsible on converting Bi into semiconductor as suggested by [11] .
4) The melting endotherm of the supersaturated solid solutions was a single sharp peak, i.e. the same as the eutectic melting.