Effect of Fermentation Process on Nutritional Composition and Aflatoxins Concentration of Doklu, a Fermented Maize Based Food ()
1. Introduction
Traditional cereal foods play an important role in the diet of the people of Africa particularly in cereal producing zones. Flour from various cereals is one of the main raw materials used in the production of popular food products with high acceptability, good storage characteristics and affordable cost [1]. These cereals are very widely utilized as food in African countries and account for as much as 77% of total caloric consumption [2]. Indeed, Cereals have a relatively better mineral profile but the availability of these minerals to human system is low [3]. Phytic acid present in considerable amount in cereal grains [4] may be partly responsible for the low digestibility of starch [5], protein [6] and low bioavailability of minerals [7,8]. A majority of traditional cereal based foods consumed in Africa are processed by natural fermentation and are particularly important as weaning foods for infants and as dietary staples for adults [9,10]. Fermentation of food grains is known to be an effective method of improving the starch and protein digestibility [11] and bioavailability of minerals [4]. Fermentation also brings down the level of antinutrients like phytic acid and polyphenols [7,12].
In Côte d’Ivoire, the indigenous fermented foods are numerous and varied. Doklu is one of these traditional fermented foods produced mainly in the southern parts of country at household level and for family consumption only. It is a snack made from maize flour and eaten at any time of the day. The people often appreciate doklu for its sour taste due to fermentation. In the preparation of doklu, cleaned and washed whole maize grains are steeped in water for 1 or 2 days, milled, mixed into a dough and left to undergo a spontaneous fermentation for 2 - 3 days by desirable microbes in the environment. One portion of the fermented dough is firstly precooked andthen shaped into balls, wrapped in maize husks and boiled for about 3 h (Figure 1).
Besides improving the digestibility, bioavailability of minerals and reducing the level of antinutrients, fermentation may also change the level of nutrients in the food grains. Indeed, during manufacture of these fermented

Figure 1. Flow diagram for production of doklu.
cereal foods, nutrients including protein and minerals are lost from the grains thereby affecting nutritional quality adversely, as reported by [13].
This paper reports the effect of spontaneous fermentation as applied at household level on the nutrients composition and aflatoxins rate of maize during the processing into doklu.
2. Materials and Methods
2.1. Sample Collection
The different samples (250 g) for this study were collected in sterile containers from 6 processors at different stages of processing (maize grains, milled flours, fermented dough and finished product doklu) in Abidjan (the economic capital of Côte d’Ivoire). All collected samples were immediately transported in an icebox directly to the laboratory for analyses.
2.2. Proximate Composition
Forty grams of samples were ground in 300 ml of distilled water in a porcelain mortar and then centrifuged at 4000 tours/min for 30 min. The pH was determined on 50 ml of the supernatant using a pH-meter (P107 Consort). Total titratable acidity was determined by titrating 30 ml of supernatant used for pH determination against 0.1 M NaOH using phenolphthalein as indicator. The total titratable acidity was calculated as percentage of lactic acid.
Organic acids of samples were before extracted and then analyzed by high performance liquid chromatography using an ion exclusion ORH-801 column (300 mm × 6.5 mm) (Interchrom, France) as achieved by [14]. Running conditions were: mobile phase, H2SO4 40 mmol·L−1; flow rate, 0.8 ml·min−1; wave length, 210 nm; room temperature (25˚C). The separated components were detected with an UV spectrophotometric detector (SPD-6A, Shimadzu Corporation, Japan).
Total sugars content were determined by the phenol sulphuric acid method according to Dubois et al. [15] and the values were expressed in g/100g of fresh dough. Total carbohydrates were determined according to the method of [16]. The contents of protein, fat, ash and moisture were determined according to the methods described in [17]. The determination of the energy value was done by calculation according to the method proposed by [18], with specific coefficients of starchy foods:
Energy value (kcal) = (3.87 × Proteins) + (4.12 × Total carbohydrates) + (8.37 × Fats).
2.3. Mineral Analyses
The method described by the Association of Official Analytical Chemists [19] was used for mineral analysis. The samples were ashed at 550˚C. The ash was boiled with 10 ml of 20% hydrochloric acid in a beaker and then filtered into a 100 ml standard flask. This was made up to the mark with deionized water. The minerals were determined from the resulting solution. Sodium [Na] and Potassium [K] were determined using the standard flame emission photometer. NaCl and KCl were used as the standards [19]. Phosphorus [P] was determined calorimetrically using the spectronic 20 [Gallenkamp, UK] with KH2PO4 as the standard. Calcium [Ca] was determined using Atomic Absorption Spectrophotometer [AAS Model SP9]. All values were expressed in mg/ 100g.
2.4. Aflatoxin Extraction from Maize Samples and HPLC Analysis
Aflatoxins were extracted after the method of [20], with slight modifications. Twenty (20) g of maize samples collected throughout the doklu process were extracted with 100 ml acetonitrile: potassium chloride (90:10, v/v). The extract was filtered and the resultant filtrate further purified with a Sepak cleanup column (Merck). Three aliquots (200 µl) of the purified extract were transferred into vials. The solvent was evaporated under nitrogen gas and the samples were stored at 4˚C. The dried samples were dissolved in 1 ml acetonitrile: potassium chloride (1:1, v/v) and filtered. An aliquot of the filtrate (300 µl) was injected into a Shimadzu HPLC system (Shimadzu, model CRB-GA, Kyoto, Japan). Separation was carried out isocratically using H3PO4 (0.33 M): acetonitrile: propanol-2 (650:400:50, v/v/v) as the mobile phase. The flow rate was maintained at 0.5 ml/min) and the fluorometric was used. Identification of aflatoxins (B1, B2, G1, and G2) in each sample was achieved by comparison with retention times of standard peaks. A series of each aflatoxin standards were used to construct a calibration curve. The equation obtained from the calibration curve was used to calculate the concentration of aflatoxins in each sample.
2.5. Statistical Analysis
The effect of processing steps on the concentration nutrient and aflatoxins in maize samples was analyzed using the analysis of variance (ANOVA) with the use of post hoc tests. Tests with P-values less than 0.05 were considered to be statistically significant.
3. Results and Discussion
3.1. Acid Production in Doklu Fermentation
The types and amounts of the main acids produced during the process of doklu production are shown in Table 1. Two acids were produced in detectable amounts, namely, lactic and acetic acids. Lactic acid rate was already higher in the maize flour, due to the 2 days soaking stage underwent by maize grains. In fact, according to reference [21], lactic acid bacteria dominate during the soaking stage of the traditional process. And as a result, a significant increase of organic acids takes place. The concentrations of these organic acids increased continuously during the fermentation and reached a maximum total of about 1.8 g/100g lactic and 0.5 g/100g acetic acids the second day of fermentation. After this period, a decrease was observed.The amounts of acids produced in this fermentation are comparable to amounts reported for many other traditional fermented foods. Reference [22] reported 0.78% lactic acid in ogi, 0.6% in kaffir beer and 1% in bussa, three spontaneous fermented cereal based products.
The pH dropped significantly during the fermentation from 5.4 in the grains to 2.2 at 48 h contrarily to the total titratable acidity which amount increased to 0.6% at the same time (Table 1). These are relatively quick variations if compared to similar fermentations, and indicates a relatively high fermentation rate. According to references [23-25], the organic acids released (e.g. lactic, acetic, propionic and butyric acids), as by-products during lactic acid fermentation, lower the pH to levels of 3 to 4 with a titratable acidity of about 0.6%. The undissociated forms of the acetic and lactic acids at low pH exhibit inhibitory activities against a wide range of pathogens. This improves food safety by restricting the growth and survival, in fermented cereal beverages, of spoilage organisms and some pathogenic organisms such as Shigella, Salmonella and E. coli [26,27]. Fermented maize dough for doklu production with pH value below 3 could have undoubtedly inhibited the growth of such organisms if they were present.
3.2. Macronutrient and Mineral Composition
The macronutrient and mineral composition of samples collected during doklu fermentation are presented in Tables 2 and 3 respectively. The moisture content values of the samples ranged between 14.2 ± 0.1 in the grains and

Table 1. Effect of fermentation stages on pH, total titratable acidity and organic acids of maize during its processing into doklu.

Table 2. Effect of fermentation stages on proximate composition of maize during its processing into doklu.
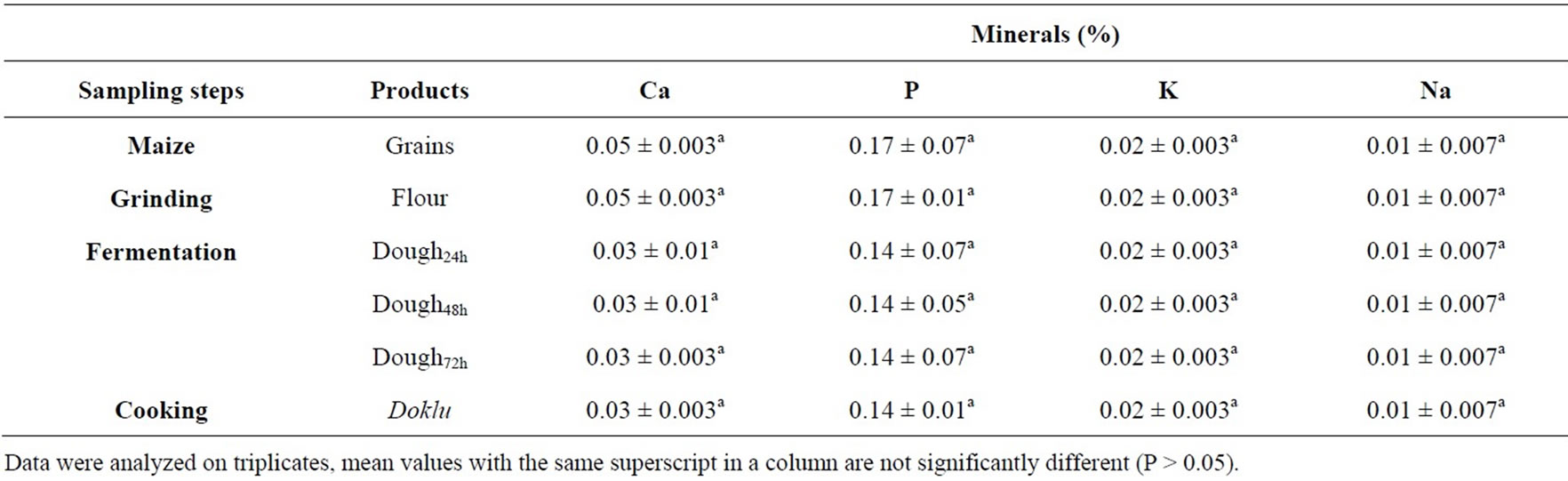
Table 3. Effect of fermentation stages on minerals composition of maize during its processing into doklu.
20.7% ± 0.1% for the final product. This variation in the moisture content is due to the fact that an amount of water is added to the maize flour to make the dough. However, the value obtained for the final product is different of those stated by [28] for kenkey, a similar maize food from Ghana. Moreover, scientific investigation has reported that low moisture content in food samples increased the storage periods of the food products [29]; while high moisture content in foods encourage microbial growth; hence, food spoilage [30].
The proteins, fatty matters and total soluble sugars contents were respectively of 8.2 ± 0.7, 0.64 ± 0.01 and 0.64% ± 0.01% in maize grains (Table 2). These values were significantly reduced during the process of doklu production. As a result of fermentation significant reducetion in crude protein and soluble sugars contents of the food may be attributed in one hand to an increase in protein catabolism by the fermenting microorganisms which leads to the escape of the by-product of metabolic deamination, i.e. ammonia and in other hand to the utilization of sugars as a carbon source. The results are similar to those reported by [31] who observed a reduction in protein content of fermented cereal-legume food mixtures by the action of bacteria and yeasts. Reference [32] also noticed a significant reduction in the protein content of pearl millet when it was fermented with L. acidophilus. According to Reference [33], fermentation may slightly alter the proximate composition substrates. A slight increase in the percentage of protein can be noted. This increase reflects the decrease of other constituents which the microorganisms might have consumed for growth. The decrease of soluble sugars and fatty matters in maize dough during doklu fermentation suggested that the fermenting microorganisms had used them as an energy source. Reduction of fat content was previously mentioned by [34] in their study on the fermentation of pear millet.
Ash content of maize seeds was 1.85% and this value was significantly affected during the various stage of processing (Table 2), whilst mineral contents and energy value remained unaffected by the fermentation process (Table 3). A similar trend was observed during the fermentation of cassava during fufu production by [35]. These authors showed that the fermentation process caused an increase in the concentration of calcium (+12%) in cassava but reductions in the levels of potassium, sodium, manganese, iron, copper, zinc and phosphorus. Data have not been published on changes in the caloric content of food as a result of fermentation processes. Generally only small changes would be expected. In processes such as tempeh production, which are aerobic, the fermentation period is too short to allow large decreases in the total lipids, carbohydrate, or protein components of the food [36].
3.3. Effect on Aflatoxins Rate
Figure 2 shows the high-performance liquid chromatogram of aflatoxins B1, G1 and G2 extracted from maize grains samples. As it could be seen on the figure, aflatoxin B2 was not detected in maize grains and consequently during all the process of doklu production.
The total amount of aflatoxins detected in the grains was 4.59 ± 0.03 µg/kg consisting in 2.52 ± 0.01 µg/kg of aflatoxin B1, 2.52 ± 0.01 µg/kg of aflatoxin G1 and 0.33 ± 0.02 µg/kg aflatoxin G2 (Table 4). Maize and maize products are known to be susceptible to contamination by fungi that produce secondary metabolites such as aflatoxins [37]. Aflatoxins have been described as extremely toxic and carcinogenic compounds, which appear to be ubiquitous in the environment [38]. The incidence and level of aflatoxin contamination in various food commodities have been monitored worldwide [19] and continues to be of great concern. Aflatoxins, particularly aflatoxin B1 (AFB1), are considered to be the most important of the mycotoxins due to their high toxicity and they are still of major concern to the feed industry and farmers as many raw materials which are used as components of animal feeds are prone to contamination [39]. Their adverse effects involves their mutagenic, carcinogenic (especially to kidneys and liver), teratogenic and oestrogen immunosuppressive effects. Aflatoxin B1 is one of the strongest carcinogens and it was included by WHO and the International Agency for Research on Cancer in the list of carcinogenic substances Group I, i.e. substances with confirmed carcinogenic effect in humans [40,41]. However, during processing of maize into doklu, important decreases in aflatoxins rates were observed (Table 4). After the 2 days soaking, the amount of aflatoxins was reduced to 1.3 ± 0.04 µg/kg (about 72%) for total aflatoxins and to 0.51 ± 0.02 µg/kg (about 80%) for aflatoxin B1. At the fermentation steps no aflatoxin was detected, involving that all the mycotoxins degraded. This study has shown that natural fermentation of maize dough for doklu production can substantially reduce the amount of aflatoxins contamining the raw material. The toxicity of the product was significantly reduced after the soaking and the first time fermentation period with progressive decrease in the pH. This is in agreement with other studies, which clearly show that lactic acid bacteria (Lactobacillus strains) efficiently remove aflatoxin B1 from the culture solution [42,43]. It has been suggested that removal of toxins is through noncovalent binding of mutagens by fractions of the cell wall skeleton of lactic acid bacteria [44]. However, other alternative mechanism of aflatoxin B1 removal has been reported, in which LAB fermentation opens up the aflatoxin B1 lactone ring resulting in its complete detoxification [45]. The lower pH of the media could also have contributed to the removal of toxins from the media as other studies have shown that treatment of LAB pellets with hydrochloric acid significantly enhanced the binding ability of the bacteria [36].
3.4. Conclusion
It may be concluded from this study that natural fermentation during the processing of maize grains into doklu resulted in reduction of some nutritional parameters, but a high increase in acidity, an important characteristic for the product safety was observed. Natural fermentation also leads to a total elimination of aflatoxins in the product. Despite the losses in some nutritional compounds, the fermented product, doklu, was found to have appreciable nutritional quality.
4. Acknowledgements
This research was made possible through funds provided by the International Foundation for Science, Sweden

Figure 2. Typical high-performance liquid chromatogram of extract of maize grains samples showing picks of aflatoxins B1, G1, G2 and their retention times.

Table 4. Effect of fermentation stages on aflatoxins content of maize during its processing into doklu.
(E/4955-1). The authors gratefully acknowledge M. SORO and M. OUATTARA from LANADA for their assistance in HLPC analysis for aflatoxins quantification.
NOTES