The effect of reduced glutathione on the chondrogenesis of human umbilical cord mesenchymal stem cells ()
1. INTRODUCTION
Cartilage degeneration is the major cause of disability and can be caused by trauma or common joint disorders such as osteoarthritis (OA) [1]. Because of their own limited repair ability, the articular cartilage lesions often lead to poor prognosis; there is no special treatment at present [2,3]. In recent years the development of tissue engineering has brought good prospects for treating cartilage defect [4]. And the selection of seed cell is the premise condition of cartilage tissue engineering. Over the years, many studies have explored the potential of different stem cell types, such as MSCs [1], to generate chondrocytes in vitro, the long-term aim being to determine if stem-cell-derived chondrocytes have the potential to repair osteoarthritic lesions [5,6], such as Human umbilical cord Mesenchymal stem cells (hUC-MSCs). hUC-MSCs are kinds of Mesenchymal stem cells from abandoned umbilical cord; they have stable properties and can long subculture. Compared with MSCs from bone marrow and peripheral blood, they have greater differentiation potential, stronger proliferation ability, lower immunogenicity, more convenient source of materials, and they have no moral restriction and easy industrialization preparation, and so on [7-12]. Therefore, they have a broad application prospect in tissue engineering and cell replacement therapy.
Sulfydryl is the main non-enzyme antioxidant in the body, including protein sulfydryl and non-protein sulfydryl. Reduced Glutathione (GSH) is an ordinary small molecule composed of three peptides in the cells, a nonprotein sulfydryl in aerobic biology. Studies have reported Glutathione could directly react with reactive Oxygen Species (ROS), such as hydroxy free radical (OH), by sulfhydryl in the non-enzyme reaction and transformed into active oxygen into easily metabolic acid substance, thus it could be cleared [13]. In enzymatic reaction of oxidation Glutathione peroxidase (GSH-px), Glutathione was oxidized to Oxidized Glutathione (GSSG), and the GSSG was converted to GSH under the action Glutathione again, playing the role of antioxidant effect [14]. In addition, cytoprotective effects of GSH were with relevant to its degradation products of glycine. Glycine has a powerful function stabilizing the cell membrane, so exogenous GSH can maintain the integrity of the membrane, to stabilize the membrane protein, reducing cell damage and promoting repair [15,16]. Therefore, the change of intracellular GSH content could reflect the oxidation resistance and cell oxidative stress state.
At the same time, GSH can coordinate the role of endogenous and external antioxidant through the direct action and participating in enzymatic reaction of some antioxidant enzyme, maintain the production and clear of free radicals, thus protecting cells from oxidation damage, and making the internal environment a stable reduction state [13,15,17]. Studies have shown that GSH could protect chondrocytes and reduce the damage of chondrocytes [18,19]. However, there have no reports about the effect of chondrogenesis of hUC-MSCs for GSH at present.
Transforming growth factor-β1 (TGF-β1) is most frequently upregulated in tumor cells, and was reported to promote the chondrogenic differentiation of MSCs [20]. The aim of this study was to investigate if GSH was able to induce chondrogenesis of hUC-MSCs in the presence of exogenous TGF-β1.
2. MATERIALS AND METHODS
All specimens (n = 4) were obtained from the fresh umbilical cord of the healthy babies after obstetric cesarean on that day in the First Affiliated Hospital of Jinan University, they were 3 - 5 cm Length, 37 - 40 weeks gestational age and had no congenital diseases; the puerpera was health, and had no hepatitis, syphilis, AIDS and other infectious diseases, puerpera and other family members were informed consent for using umbilical cord in experimental studies.
2.1. Isolation and Subculture of hUC-MSCs
Neonatal umbilical cord was cut into pieces and digested by 0.2% collagenase II (Sigma company) at 37˚C for 2.5 h in shaking bath, the cells were collected and resuspended with DMEM/F12 medium (Gibco company) contained 10% FBS (Gibco company) after centrifugation and seeded at 2 × 103 cells/cm2 in a new T25 culture flask. The medium was changed after three days and the nonadjacent cells were removed, the cells obtained were primary cells. When the cells had grown to 90% confluence, digested with 0.25% trypsin (from Sigma), the supernatant was discarded after centrifugation, cells were resuspended with DMEM/F12 medium and the cell number and viability were assessed with the trypan blue assay (Sigma), The cells were seeded at 2 × 103 cells/cm2 in a new T25 culture flask at 37˚C under a 5% CO2 atmosphere, continued to culture and observed the cell growth.
2.2. Evaluation of Cell Cycle
hUC-MSCs were harvested, the cell number was adjusted to 5 × 105 cells/ml, and the cells were suspended with 1 ml 70% (v/v) 4˚C cold ethanol, then fixed for 24 h in the 4˚C refrigerator; The cells were suspended with 1 ml Presidium Iodide (PI 50 μg/ml) (supplemented with 10 μg/ml RNAase A) after discarding the suspernatant, incubated for 20 min at 4˚C in the dark, then detected using a hemocytometer, 104 cells were obtained by the Cell-Quest software and the cell cycle was detected by ModiFit.
2.3. Evaluation of the Potential for Multilineage Differentiation of hUC-MSCs
2.3.1. Osteogenic Differentiation of hUC-MSCs
hUC-MSCs were treated according to the experimental procedure of Osteogenic Differentiation kit (CYAGEN Company).
2.3.2. Adipogenic Differentiation of hUC-MSCs
hUC-MSCs were treated according to the experimental procedure of Adipogenic Differentiation kit (CYAGEN Company).
2.3.3. Chondrogenesis of hUC-MSCs
hUC-MSCs were cultured in 6-well tissue culture plates pre-coated with Gelatin Solution at 3 × 104 cells/ml and incubate at 37˚C in the 5% CO2 humidified incubator. When cells were approximately 80% - 90% confluent, they were aspirated off the DMEM/F12 medium from each well and added 2 ml hUC-MSCs chondrogenic differentiation Medium (DMEM high sugar medium supplemented with 0.1 μM dexamethasone, 50 μg/ml vitamin C, 6.25 μg/ml insulin, 6.25 μg/ml transferring, 10 ng/ml TGF-β1, 5% FBS). After three weeks differentiation, the cells were fixed and stained with Alcian Blue overnight, then visualized under light microscope and they images were captured.
2.4. Chondrogenic Differentiation of hUC-MSCs
There were three experiment groups. hUC-MSCs in growth period were seeded at a density of 4 × 105 cells/well in sterile coverslips pre-coated poly-lysine in four 6-well plates, and placed in a 5% CO2 incubator at 37˚C. When the cells had grown to 80% confluence, the basic chondrogenic induction medium (DMEM high sugar medium supplemented with 0.1 μM dexamethasone, 50 μg/ml vitamin C, 6.25 μg/ml insulin, 6.25 μg/ml transferring, 10 ng/ml TGF-β1, 5% FBS) was added in experiment group 1 (Group B), the basic chondrogenic induction medium +0.5% DMSO (DMEM high sugar medium supplemented with 0.1 μM dexamethasone, 50 μg/ml vitamin C, 6.25 μg/ml insulin, 6.25 μg/ml transferring, 10 ng/ml TGF-β1, 5% FBS and 0.5% DMSO) were added in the second experiment group (Group BD), and the basic chondrogenic induction medium +0.5% DMSO +500 µM GSH (DMEM high sugar medium supplemented with 0.1 μM dexamethasone, 50 μg/ml vitamin C, 6.25 μg/ml insulin, 6.25 μg/ml transferring, 10 ng/ml TGF-β1, 5% FBS and 0.5% DMSO dissolved 500 µM GSH) were added in the third experiment group (Group BDG). While the DMEM/F12 medium was added in the control group (hUC-MSCs group) and changed every 3 days. The morphology was observed every 2 days in four groups. The culture lasted for 21 days.
2.5. Toluidine Blue Staining
After induced culture for 21 days, the cell climbs were washed in PBS, fixed with paraformaldehyde for 10 min and stained with Toluidine blue for 30min; then washed again until the background was clear, dehydrated, observed and photographed under the microscope.
2.6. Immunofluorescence Technique
The cell climbs were washed in PBS twice, fixed with paraformaldehyde for 10 min, washed thrice, and treated with 0.5% Triton X-100 (prepared with PBS) for 10 min, then washed thrice again, and incubated with a normal goat serum for 30 min; washed twice, and incubated with primary antibody overnight at 4˚C (solubility of 1:100). Washed thrice and incubated with secondary antibodies which were labeled with cy3 in a dark place at room temperature for 60 min (solubility of 1:64). The cells were washed twice in PBS and thrice in deionized water, coated with fluorescent anti-quencher and examined under an immunofluorescence microscope.
2.7. Quantitive Detection of Type II Collagen and Glycosaminoglycan
Culture medium and cells after induction for 7, 14, 21 days respectively were collected for the quantitative detection of COL2-A1 and GAG. hUC-MSCs with the same days were used in control group. Quantitative detection of COL2-A1 was done by hydroxyproline method. The steps of each sample were in accordance with the instructions of hydroxyproline kit (digestion method). Quantitative detection of GAG was done by alcian blue method [21]. Each specimen was digested with papain, and the alcian blue was added in the digestion product then their absorbance values were obtained in the microplate reader (TZCAN, Austria) at 600 nm.
2.8. Quantitative Real-Time PCR
hUC-MSCs and chondrocytes after chondrogenic induction were detected by Quantitative real-time PCR. cDNA as the template of qPCR was synthesized from 1 µg total RNA. COL2-A1 and GAG mRNA level were detected by RT-PCR, and GAPDH was chosen as internal control. The results of all samples were done relative quantitative analysis based on the expression of GAPDH. Data were analyzed using △△Ct method [22], relative copy number = 2−ΔΔCt = 2−(△Ct target−△Ct GAPDH) = 2−[(Ct target)−Ct GAPDH)−(Ct control−Ct GAPDH)], each sample was analyzed in triplicate. PCR was performed using specific primers (Invitrogen company) designed from the published sequence of each cDNA like Table 1.
2.9. Statistical Analysis
Data were reported as means ± SD (x ± s), and were analyzed statistically using the SPSS 17.0 software.
3. RESULTS
3.1. The Detection of Cell Cycle by Flow Cytometry
The results showed that (Figure 1) (77.08 ± 0.84)% of hUC-MSCs were in the G0/G1 phase, the rest of them
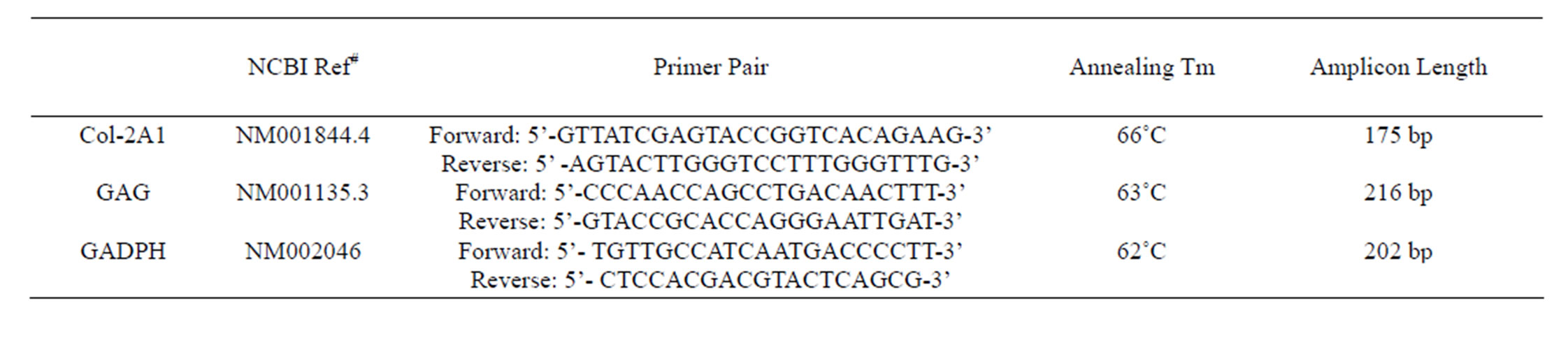
Table 1. Real-time quantitative PCR primer sequences.

Figure 1. Cell cycle of hUC-MSCs. 78% of hUC-MSCs were in the G0/G1 phase.
were in the proliferative phase. That meant hUC-MSCs have the ability of multilineage differentiation.
3.2. Evaluation of the Potential for Multilineage Differentiation of hUC-MSCs
After cultured for 21 days, the hUC-MSCs exposed to osteogenic medium were strongly positive to Alizarin red staining. The hUC-MSCs in the control group were negative to Alizarin red staining (Figures 2(a) and (b)). Adipogenesis was confirmed by the presence of neutral lipid vacuoles (lipid droplets) that were strongly positive to Oil Red O staining at day 14. While, there was very shallow or little color in the control group (Figures 2(c) and (d)). The cartilaginous phenotype of the induced cells was confirmed by Alcian blue staining at day 21 (Figures 2(e) and (f)). And the color of Alcian blue staining in the hUC-MSCs group, negative control, was light blue.
3.3. Observation of Chondrogenic Differentiation of hUC-MSCs in the Microscope
After induction for 14 days, the hUC-MSCs gradually turned into circular, triangular, polygonal, and began to grow exhibiting an aggregated morphology under microscope in Group B, Group BD and Group BDG (Figures 3(a)-(d)). The phenomena in Group B and Group BD were basically consistent, while the above phenomenon in Group BDG was more evident.
3.4. The Detection of GAG by Toluidine Blue Staining and COL2-A1 by Immunofluorescece Staining
The cells after induction were strongly positive in Group B, Group BD and Group BDG, the extracellular matrix was stained purple blue (Figures 3(e)-(h), compared with hUC-MSCs group. The immunofluorescece staining results showed that the cells grew intensively; the cells
and the extracellular matrix showed a red fluorescence in Group B, Group BD and Group BDG, while the hUCMSCs were weakly positive expression in the negative control group (Figures 3(i)-(l)).
3.5. The Detection of COL2-A1 and GAG Contents
The result showed the GAG content of hUC-MSCs group was far lower than Group B, Group BD and Group BDG at every time points, and there were obvious statistical significance (P < 0.05). However, the GAG content of Group BDG was higher than Group B and Group BD, and there were noticeable statistical significance (P < 0.05) (Figure 4(a)).
The results of the content of COL2-A1 show that, the content of hydroxyproline in hUC-MSCs group were lower than Group B, Group BD and Group BDG at day 7, day 14 and day 21, there was no difference between
Group B and Group BD, but there were obvious statistical significance among Group BDG and other groups (P < 0.05) (Figure 4(b)).
3.6. Quantitative Real-Time PCR
The results showed that there were significant differences in the expression of COL2-A1 and GAG mRNA of the cells in Group B, Group BD and Group BDG (P < 0.05), compared with the hUC-MSCs group. And the expression of Col2-A1and GAG mRNA of the cells in Group BDG was obviously higher than that found in Groups B and BD (P < 0.05) (Figure 5)
4. DISCUSSIONS
In the research, we have investigated the effect of GSH on the chondrogenic process of hUC-MSCs. Our study has shown that by adding the GSH to basic chondrogenic medium, we can direct the differentiation of hUC-MSCs down a chondrocyte lineage. To our knowledge this is the first study to demonstrate chondrogenic induction via GSH, in the presence of growth factors such as TGF-β1.
In this study, hUC-MSCs were isolated by collagenase II digestion which could be adhered to the plastic and showed long spindle or polygonal, parallel growth or swirly growth under the microscope. The Alizarin Red staining of hUC-MSCs was positive after osteogenic induction. The oil red O staining of hUC-MSCs was positive after adipogenic induction, showing that fat granule had secreted. The Alcian blue staining of hUC-MSCs after chondrogenic induction was also positive, demonstrating that a number of GAGS had produced. The above phenomenon indicated that hUC-MSCs could be induced into bone, fat and cartilage, had the ability to multi-differentiation, in line with the characteristics of mesenchymal stem cells, consistent with bone marrow-

Figure 4. The result of quantitative detection of the content glycosaminoglycan (a) and type II collagen (b). *P < 0.05, there were significant differences; **P < 0.05, there were very significant differences. The biologically replicated data is represented as mean ± standard deviation; n = 4.

Figure 5. The expression level of GAG and COL2-A1 mRNA. *P < 0.05, there were significant differences; **P < 0.05, there were very significant differences; ***P < 0.05, there were more obvious differences. The biologically replicated data is represented as mean ± standard deviation; n = 4.
derived mesenchymal stem cells [23].
On the basis of previous studies, our team came to such conclusions: GSH can be dissolved in the dimethyl sulfoxide (DMSO), and 0.5% DMSO had no effect on the proliferation of hUC-MSCs. At the same time, 500 µM GSH, dissolved in the DMSO, had no toxicity to hUC-MSCs. Therefore, we chose 500 µM GSH in our research.
Collagen accounts for 60% - 80% in cartilage tissue, wherein the type II collagen is 90% in a total collagen, the major collagen constituting articular cartilage [23,24]. Hydroxyproline is the characteristic amino acid of type II collagen, the higher the hydroxyproline content is, the higher the type II collagen content is. GAG, surrounded by collagen rack, accounts for 20% - 40% of the weight of cartilage tissue [23]. Under specific differentiation-inducing conditions, hUC-MSCs can differentiate into cartilage-like cells and express chondrocyte-specific genes, COL-2A1and GAG, the resulting cartilage matrix established phenotype [25].
GSH was added in the basic chondrogenic induction medium. After 14 days, there had similar phenotype changes of hUC-MSCs in Group B and Group BD, indicating that it had no influence or side effects on chondrogenic induction of hUC-MSCs for 0.5% DMSO. The results of toluidine blue staining and immunofluorescence technique revealed that Toluidine blue staining and immunofluorescence staining were all positive in Group B, Group BD and Group BDG, showing that abundant GAG and Type II collagen had secreted. The cell morphology changes and extracellular matrix secretion had no obvious difference in each group.
Interestingly, we found that the content of GAG and Type II collagen relatively and obviously increase in Group BDG, compared with Group B and Group BD (P < 0.05), further qPCR result suggested that their mRNA expression of genes also increased. And there had significant statistical significance (P < 0.05).
5. CONCLUSION
In summary, our results show that GSH can direct hUC-MSCs to undergo chondrogenesis of hUC-MSCs in the absence of exogenous TGF-β1. The process of chondrogenesis on the GSH appeares similar to that which occurs in standard pellet cultures. At the same time GSH possibly enhances the antioxidant ability of cells, thus making cells be protected well. However, the facts that why and how GSH promotes the chondrogenesis of hUC-MSCs are still unknown, therefore, we need to research deeply.
NOTES
#Co-authors.
†Corresponding authors.