1. Introduction
Oxidation is the transfer of electrons from one atom to another and represents an essential part of aerobic life and our metabolism, since oxygen is the ultimate electron acceptor in the electron flow system that produces energy in the form of ATP. However, problems may arise when the electron flow becomes uncoupled (transfer of unpaired single electrons), generating free radicals. The uncontrolled production of oxygen-derived free radicals is hostile and damaging to cells and their functions. Dietary antioxidant compounds (polyphenolic compounds, vitamin, and ascorbic acid) and endogenous enzymes (superoxide dismutase, glutathione peroxidase, and catalase) can protect the organism against oxidation damage. When the mechanisms of antioxidant protection become unbalanced by some factors, deterioration of physiological functions may result in degenerative or pathological processes such as aging, cancer, coronary heart diseases, etc. The use of traditional medicine is widespread and plants still present a large source of natural antioxidants that might serve as leads for the development of novel drugs [1]. There is a great interest in the search for new antimicrobial drugs also from nature, because of the resistance that pathogenic build against antibiotics [2]. In many parts of the world medicinal plants are used for antibacterial, antifungal, and antiviral activities. These plant extracts were used as a source of medicinal agents to cure urinary tract infections, cervicitis vaginitis, and gastrointestinal disorders (Caceres et al., 1990).
In the present study, two medicinal plants from Aizoaceae family were screened for their antibacterial and antioxidant activities. The species Mesembryanthemum crystallinum is characterized by the presence of antioxidants enzymes such as ascorbate peroxidase, superoxide dismutase and catalase (Selzak et al. 2002). Historically, physicians used leaf juice to soothe inflammation of the mucous membranes of the respiratory or urinary system. In Europe, the fresh juice has been used to treat water retention and to painful urination and to soothe lung inflammation [3].
The species Mesembryanthemum edule or Carpobrotus edulis, was traditionally used as a medicinal plant [4]. The leaves contain flavonoids (rutin, neohesperidin, hyperoside), catechin, ferulic acid and catechol tannins [4]. According to Roberts [5] the leaf juice is also effective in soothing itching caused by spider and tick bites. The leaves also contain an astringent antiseptic juice which can be taken orally for treating sore throat and mouth infections [6]. The fresh leaf juice and aqueous extract of dried leaf showed in vitro antimicrobial activity against Staphylococcus aureus and Pseudomonas aeruginosa [4]. The antibacterial activity of Carpobrotus edulis (crude extract) can be attributed to the presence of different flavonoids as well as tannins [4], but we still need more information about the medicinal properties of the two plant growing on Tunisian costs particularly Mesembryanthemum crystallinum.
In the present paper, we investigate the potential antioxidant and antibacterial properties of both plants collected from Tunisian biotope, in order to evaluate their medicinal potentiality and their future industrial uses.
2. Materials and Methods
2.1. Plant Material
The leaves of the two plants Mesembryanthemum crystallinum and Carpobrotus edulis were collected from their native biotope (Monastir Falaise) at the period of December 2007. The identification of the two species was done by the department of the botany (Faculty of Pharmacy, Monastir).
2.2. Preparation of the Extract
500 g of fresh leaves were chopped into small parts in a blender in the presence of 250 ml of distilled water, boiled for 15 min and followed by filtration.
2.3. Total Flavonoids Determination
The flavonoids are determined according to Chang et al. [7] with modification, through aluminium chloride colorimetric method. 250 µl of plant extract (0.01%) was mixed with 75 µl of NaNO2, 150 µl AlCl3 (10%), 500 µl NaOH (1 N) and 2.5 ml of distilled water. It remained at room temperature for 5 min; the absorbance reaction of the mixture was measured at 510 nm. The calibration curve was prepared by preparing quercitin solutions at 50 to 500 µg/ml.
2.4. Total Phenols Determination
The total phenolic compounds were determined as described by the modified methods of Taga et al. [8], using Folin-Ciocalteu reagent. 100 µl of plant extract (0.01%), Folin-Ciocalteu reagent (500 µl) and sodium carbonate (2 ml, 2%) were thoroughly mixed and kept at room temperature for 30 min before the absorbance at 720 nm was measured. Total phenolic concentration was determined using gallic acid as standard.
2.5. DPPH Free Radical Scavenging Activity
Antioxidant activity was determined by using a stable free radical (1,1-diphenyl-2-picrylhydrazyl) DPPH [9]. DPPH solution was prepared at the concentration of (0.024 mg/ml DPPH in ethanol). During assay 1 ml of the crude extract was mixed with 1 ml DPPH solution. The mixture was incubated in the room temperature for 30 min; absorbance was recorded at 517 nm (Cam spec M230/330 UV visible spectrophotometer, United Kingdom). Butylated hydroxytoluene (BHT), butylated hydroxyanisol (BHA) and Vitamin C were used as a standard for the investigation of the antiradical activity:
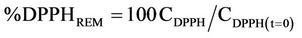
where CDPPH(t=0) is the initial DPPH concentration and CDPPH is the DPPH concentration at the steady state.
2.6. Antibacterial Activity
Escherichia coli (ATCC 25922), Pseudomonas aeruginosa (ATCC 27853) and Staphylococcus aureus (ATCC 25923) are bacterial strains employed in the screening. Disc diffusion method was used for the determination of inhibition zone, the broth dilution method for the estimation of the Minimal Inhibitory Concentrations (MIC) and the Minimal Bactericide Concentrations (MBC). To obtain 107 bacteria/ml, microorganisms were grown at 37˚C for 3 hours under agitation and we realized dilution 1/10 for each tube to obtain 106 bacteria/ml [10]. For the determination of the antibacterial activity, different concentrations of the lyophilized plant extract were employed (100, 200, 300 and 600 mg).
2.6.1. The Method of Disc Diffusion
In this method, the presence or the absence of inhibition zone around the Wattman paper discs (6 mm), impregnated with microbial suspensions, can indicate the sensibility or resistance of the bacteria to the plant extract. Discs were deposited on the surface of inoculated Agar MH plates, vancomycine and ceftazidine were employed as a positive control and plates with bacteria were incubated for 24 h at 37˚C.
2.6.2. Minimal Inhibitory Concentration
For the determination of the minimal inhibitory concentration, we used 96-well microtitre plates. 50 µl of bacterial suspension at a density of a 106 bacteria/ml was inoculated in each well. The microtitre plates were incubated at 37˚C for 18 h. Minimum inhibitory concentrations (MICs) were calculated based on the density of growth control and lowest extract concentrations that resulted in 80% reduction in growth compared with that of the extract-free growth control. 100 µl DMSO (10%) solution with 50 µl of culture medium were assayed as the negative control.
2.6.3. Minimal Bactericide Concentration (MBC)
The determination of the MBC was done in the same time to that of the minimal inhibitory concentration; we realized a dilution from 10 to 10–5 of the microbial suspensions to find out the number of bacteria. The different dilution and the microbial suspensions were deposited in 5 cm on the surface of agar plates. For each well with no visible bacterial growth, 10 µl (bacteria + plant extract) was deposited in 5 cm on the surface of agar plates. After incubation for 24 h at 37˚C, we determined the bacterial number.
3. Results
3.1. Antioxidant Activity
The evaluation of the antioxidant activity between the two plant extracts is showed in Figure 1. We obtained a higher antioxidant activity in Carpobrotus edulis extract (2 mg/ml induces a 94.64% ± 0.45% of DPPH inhibition) in comparison with M. crystallinum extract (2 mg/ml induces a 75.5% ± 1.07% of DPPH inhibition).
Also we used a synthetic antioxidant BHT as standard for the determination of the antioxidant activity. Carpobrotus edulis extract showed a high antioxidant activity in comparison with BHT for a concentration up to 1 mg/ml.
3.2. Antibacterial Activity
3.2.1. The Method of Disc Diffusion
The results (Table 1), showed that the extract of Carpobrotus edulis induces an inhibition zone only for Staphylococcus aureus with a diameter of 17 mm. The data showed an absence of inhibition zone against Esherichia coli and Pseudomonas aeruginosa. The extract from M. crystallinum has no inhibition zone in the different strains tested in this study with disc diffusion method. In comparison with antibiotics discs, Carpobrotus edulis extract induce the same results as vancomycine (the inhibition zone against Staphylococcus aureus: 17 mm).
3.2.2. Minimal Inhibitory Concentration
The determination of the antibacterial activity in the two plant extracts by the Minimal inhibitory concentration showed the presence of activity against the three bacterial strains tested (Table 2). A high antibacterial activity was obtained against Staphylococcus aureus (MIC 0.4%) in comparison with the activity obtained against Pseudomonas aeruginosa (MIC 6.6%) and Esherchia coli (MIC 1.6%).
In the presence of Mesembryanthemum crystallinum extract, we obtained antibacterial activity at a concentration of 600 mg (Table 3). The higher activity was obtained against Pseudomonas aeruginosa (MIC 6.6%) in comparison with that obtained against Esherchia coli (MIC 19.8%) and Staphylococcus aureus (MIC 12.5%).
3.2.3. Minimal Bactericide Concentration Determination
This method corresponds to the lowest concentration of
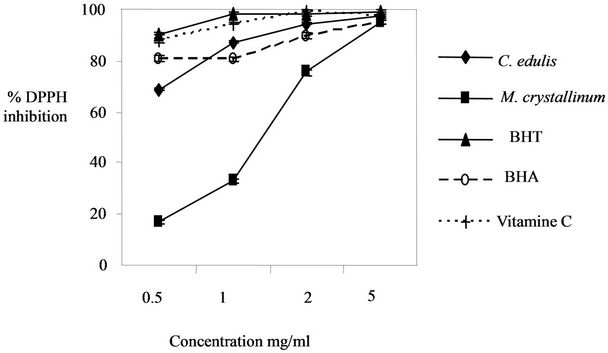
Figure 1. Comparison of the antioxidant activity in the two plant extracts.

Table 1. In vitro antibacterial activity of the two plant extracts. Vancomycine and ceftazidine are the two antibiotics used as positive control.
plant extract that is able to kill 99.99% of the bacterial strains tested. Carpobrotus edulis extract, showed high bactericide activity (Table 4) against Escherchia coli (MBC 50%). Same minimal bactericide concentration was obtained against the two other bacterial strains depending on the extract concentration; Pseudomonas aeruginosa (MBC 100%) and Staphylococcus aureus (MBC 100%). The Mesembryanthemum crystallinum extract showed bactericide activity against the two bacterial strains Escherchia coli (MBC 100%) and Pseudomonas aeruginosa (MBC 100%) (Table 4).
3.3. Determination of Flavonoids Content
The determination of the flavonoids in the plant extracts (Figure 2), showed a higher content in in C. edulis (116.16 ± 3.34 µg/mg) in comparison with M. crystallinum (4.85 ± 0.9 µg/mg).
3.4. Determination of the Total Phenols Content
The results of the phenols content are shown in the Figure 3. A higher phenols content was obtained in C. edulis extract (104.69 ± 0.48 µg/mg) in comparison with M. crystallinum (23.89 ± 0.27 µg/mg).
4. Discussion
Many methods were used to evaluate the antiradical/ antioxidant activity in plant tissues. In this study, the DPPH method was used for the determination of antiradical activity and the synthetic antioxidant (BHT and BHA) and vitamin C as standard. The DPPH test is already reported to evaluate in relatively short time the antiradical activity [11]. The absorbance decreases as a result of a colour change from purple to yellow as the radical is scavenged by antioxidants through donation of
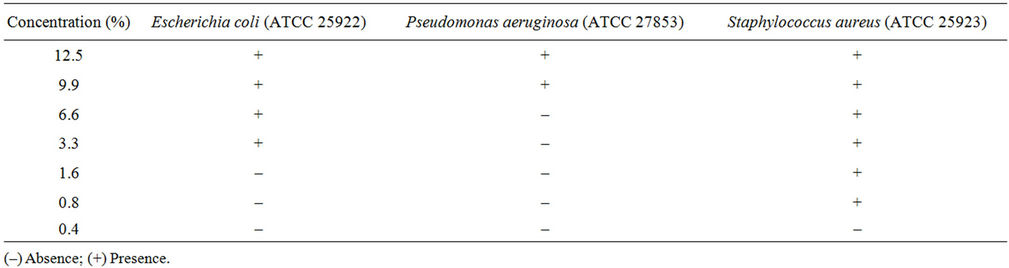
Table 2. Minimal Inhibitory Concentration (MIC) of C. edulis extract.
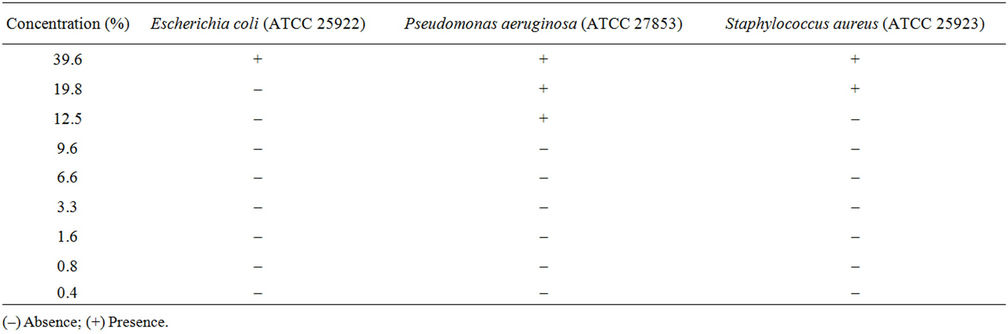
Table 3. Minimal Inhibitory Concentration (MIC) of M. crystallinum extract.

Table 4. Minimal Bactericide Concentration (%) in C. edulis and M. crystallinum extracts.

Figure 2. Flavonoids contents in the two plants extracts.
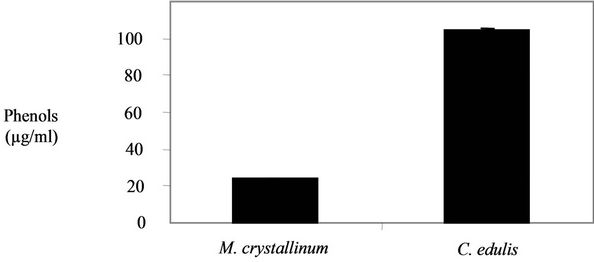
Figure 3. Phenols contents in the two plants extracts.
hydrogen to form the stable DPPH-H molecule [12]. The DPPH method can be used for solid or liquid samples and is not specific to any particular antioxidant component, but applies to the overall antioxidant capacity of the sample [13]. The two plants extracts exhibited at a concentration of 2 mg/ml more than 70% scavenging activity. C. edulis showed higher antiradical activity (2 mg/ml induces a 94.64% ± 0.45% of DPPH inhibition) than that of M. crystallinum (2 mg/ml induces a 75.5% ± 1.07% of DPPH inhibition). C. edulis and M. crystallinum extract showed low antioxidant activity in comparison with BHT and vitamin C. The antioxidant activity in C. edulis extract (concentration up to 2 mg/ml) was higher than the synthetic antioxidant BHA. The results of the antioxidant activity in the two plant extracts can be related to the phenol contents. In fact, C. edulis contain a high amount of flavonoids (116.16 ± 3.34 µg/mg) and phenols (104.69 ± 0.48 µg/mg) in comparison with M. crystallinum which contain 4.85 ± 0.9 µg/mg of flavonoids and 23.89 ± 0.27 µg/mg of phenols. Flavonoids are a group of polyphenolic compounds [14] common in leaves of plants and protect them against the damaging effect caused by UVradiation and antimicrobial infections [15]. Common family members of flavonoids include flavones, flavanes, flavonols, catechins, and anthocyanins [16]. Different flavonoids and phenolic compounds react with free radical to reduce the degradation of membranes by preventing the reaction between free radicals and phospholipids [15]. They can also be used as antioxidants and in vitro as enzyme inhibitors [17]. Flavonoids antioxidants function as scavengers of free radicals by rapid donation of a hydrogen atom to radicals [39]. Many phenolics, such as flavonoids, have antioxidant capacities that are much stronger than those of vitamin C and E [18].
Many studies on medicinal plants confirm the relation between the antioxidant activity and the presence of polyphenolics content. For example, Oke and Hamburger [19] demonstrate that the medicinal Nigerian plants contain antioxidants confirmed by DPPH test and the major components are polyphenolics compounds. In the medicinal plant Prunella vulgaris, the organic fraction posses a marked antioxidant activity confirmed by the presence of phenolic acids and other phenolic compounds [19]. The sweet potatos (Ipomoea batatas) ethanolic extract (100 mg dry mater/ml) had 41.5% DPPH scavenging activity directly related to the total amount of phenolic and flavonoid found in the sweet potato extract [1]. Pourmorad et al. [14] demonstrate a high antioxidant activity in the Iranian medicinal plant Mellilotus officinalis extract (0.1 mg/ml induces 94.3 ± 0.6), 4 times greater than the synthetic antioxidant BHT this was correlated to the presence of polyphenols in the plant extract. Mellilotus officinalis extract had higher phenol content (289.5 ± 5 mg/g) in comparison with our plants results, but lower flavonoids content in comparison with our finding with C. edulis extract.
In this study, we used the two plant extract M. crystallinum and C. edulis for the determination of the antibacterial activity. The leaf juice from C. edulis is more widely utilized than that of C. acinacformis or C. delicious as a traditional remedy [20] for a wide range of fungal and bacterial infections [17] and treatment of sinusitis, diarrhea, infantile eczema, tuberculosis and other internal chest conditions. In M. crystallinum, most of the studies conducted confirm the resistance of this plant to insects, fungal, bacterial or viral based diseases [21]. This resistance can be due to the presence of molecules which have medicinal properties such as antibacterial activity. Our results showed that the antibacterial activity was related to the plants extract concentration and to the bacterial strains. The potential antibacterial activity of C. edulis was better than M. crystallinum against Staphylococcus aureus and Escherichia coli. M. crystallinum had a better antibacterial activity against Pseudomonas aeruginosa than C. edulis. Martins et al. [4] showed that a methanol extract from C. edulis (native to Portugal) was inactive against Staphylococcus aureus, but inhibit the growth of these bacteria once phagocyted by monocyte derived human macrophage. C. edulis extract from South Africa has been shown to have a high antibacterial activity [4] which has contributed to the presence of flavonoids and tannins. In vitro assays of two other South African Carpobrotus species [22] it showed that aqueous and ethyl acetate fractions obtained from both C. muirii and C. quadrifidus inhibited the growth of Staphylococcus aureus and Mycobacterium smegmatis, but had no effect on that of Candida albicans or Pseudomonas aeruginosa. However, not many study examined the antibacterial properties of the halophyte plant M. crystallinum, most of the research focused on its responses to abiotic stresses. It was confirmed in the most of the study that this plant showed a high resistance to bacterial based diseases [21]. This was proved in the present study by the antibacterial activity and by a bactericide potential of both plants extracts. Our results confirm the ethnobotanical survey of the two plants, Carpobrotus edulis leaf juice is more widely utilized than that of C. acinacformis or C. delicious as a traditional remedy [20] for a wide range of fungal and bacterial infections [17]. Mesembryanthemum crystallinum is demulcent and diuretic and was used to treat water retention and painful urination and to soothe lung inflammation [3]. There is no much information available on the phytochemical and pharmacological properties of M. crystallinum, most of studies focused on its responses to abiotic stress as a halophyte plant.
5. Conclusion
The results of the present study confirm the high antioxidant activity in the two plant extracts which exceed 70% of DPPH inhibition. Antioxidant activity was determined by using a stable free radical 1,1-diphenyl-2- picrylhydrazyl (DPPH). The absorbance decreases as a result of a color change from purple to yellow as the radical is scavenged by antioxidants through donation of hydrogen to form the stable DPPH-H molecule. Antibacterial activity was also detected in the two plant extracts against the bacterial strains tested Staphylococcus aureus, Esherichia coli and Pseudomonas aeruginosa. Antibacterial activity was determined by disc diffusion method, minimal inhibitory concentration and minimal bactericide concentration methods. In this report, the antibacterial and antioxidant properties of Carpobrotus edulis extract approved the traditional uses of this plant. We also validate the biological activity of Mesembryathemum crystallinum extract, because most of studies conducted on this plant focused on its physiological response to abiotic stress.
6. Acknowledgements
The authors gratefully acknowledge Mr. Mahmoud Amor (Laboratoire de Pharmacognosie, Biologie Végétale, Pharmacologie Faculté de Pharmacie Monastir Tunisie).
NOTES