The Role of Valence Electron Concentration on the Structure and Properties of Rapidly Solidified Sn-Ag Binary Alloys ()
1. Introduction
Valence electron concentration (VEC) plays an important role on the structure and properties of alloys. This quantity indicates the number of all valence electrons in the alloy per number of atoms. Hume-Rothery found that definite structures of compounds arise in certain ranges of the valence electron concentration [1]. These compounds are called Hume-Rothery compounds or electron compounds. Also [2,3] found that the axial ratio c/a of the β-Sn tetragonal unit cell increases by increasing VEC and decreases by decreasing VEC. This observation is confirmed recently by [4]. The increase in the axial ratio with decreasing VEC was explained qualitatively by [2,5] on the basis of the interaction between Fermi surface and Brillouin zone. When the Brillouin zone boundary touches the Fermi sphere, the structure corresponding to the zone will be stabilized. As a result, a pseudo-gap of density of states around the Fermi level will arise. This is the HumeRothery condition of phase stability i.e., KB = 2kF where KB is the diameter of Brillouin zone and kF is the radius of Fermi sphere. Also it is found by [6] that the most important factor for the formation of stable quasicrystals is the valence electron concentration.
Fermi parameters such as Fermi energy EF, radius of Fermi sphere kF Fermi velocity vF, and the diameter of the Brillouin zone KB can be calculated from the following equations;
 ,
, 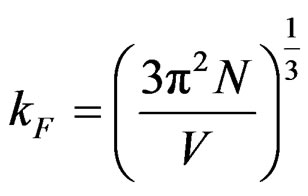 ,
,  ,
, 
where N/V is the total number of electrons per unit volume in the alloy, m is the effective mass, ħ is the reduced Plank’s constant and dhkl is the interplanar distance.
Another example of the importance of VEC is the connection which has been observed by [4,7] between Young’s modulus and the axial ratio c/a of the tetragonal unit cell of b-Sn in which Young’s modulus increases by increasing the axial ratio. Also it is found that the resistivity decreases by increasing c/a. Therefore the objective of the present work is to study the role of valence electron concentration on the structure and properties of rapidly solidified Sn-Ag binary alloys using single roller melt-spinning technique. Rapid solidification has been used in the present work to prevent rejection of extra solute atoms and thus prevent precipitation, from a solid solution.
2. Experimental Procedures
A group of binary Sn-xAg alloys (x = 0.5, 1.5, 2.5, 3.5, 4.5, 5.5 and 6.5 wt%) have been produced by a single copper roller melt-spinning technique.
Required quantities of the used metals were weighed out and melted in a porcelain crucible. After the alloys were molten, the melt was thoroughly agitated to effect homogenization. The casting was done in air at a melt temperature of 800˚C. The speed of the copper wheel was fixed at 2900 r.p.m. which corresponds to a linear speed of 30.4 m·s–1. X-ray diffraction analysis is carried out with a Shimadzu x-ray diffractometer (DX-30), using Cu-Ka radiation with a Ni-filter (l = 0.154056 nm). Differential thermal analysis (DTA) is carried out in a Shimadzu DT-50 with heating rate 10 K/min. The measurement of resistivity is carried out by the double bridge method [8]. Young’s modulus was measured by the dynamical resonance method [9]. Vickers microhardness number (HV) is measured using the FM-7 microhardness tester.
3. Results and Discussion
3.1. Structure
Figure 1 shows the x-ray diffraction patterns for asquenched melt-spun Sn-Ag alloys. The structure of Sn- 0.5Ag alloy is single solid solution of Ag in Sn (Figure 1(a)). The solid solubility of Ag in Sn for conventional method is about 0.02 wt% at room temperature according to the equilibrium phase diagram [10]. This means extension of solid solubility from 0.02 to 0.5 wt% Ag. The Ag atoms are trapped in Sn lattice in excess of equilibrium concentration due rapid solidification. For Sn- 1.5Ag alloy an intermetallic compound Ag3Sn is formed (Figure 1(b)). From 2.5 to 5.5 wt% Ag the number and intensity of peaks due to intermetallic compound Ag3Sn increase which means that its proportion increases (Figures 1(c)-(f)). The intermetallic compound Ag3Sn is orthorhombic with space group (Pmmn) and lattice parameters a = 5.968 A, b = 4.7802 and c = 5.1843 A.