Various Routes to Synthesise 3-Thioxo-1,2,4-Triazine-5-One Derivatives as Antimicrobial Agents ()
1. Introduction
Nitrogen containing heterocyclic compounds have a huge interests by synthetic chemists, due to their various pharmacological activities [1] [2] . Triazine derivatives expose a wide pharmaceutical activity such as antiviral [3] [4] , antiproliferative and antitumor [5] . Moreover, their anti-HIV [6] [7] , anti-cancer [8] , anti-microbial [9] , antileukemic and anti-convulsant activity were in vitro supported [10] [11] .
For the significant impact of 1,2,4-triazine moieties at the synthesis area of biologically active compounds 3-thioxo-1,2,4-triazin-5-one derivatives were targeted. It has a great attention mainly due to their medicinal properties, as well as biocidal molluscicidal agent against some snails [12] . In addition, most of functionally 1,2,4-triazones form stable metal-complexes with metal ions [13] . Also, the reactivity of 3-thioxo-1,2,4-triazin-5-ones depends onto the active groups, type of activation nature of substitution, leaving moities, character of nucleophile engaged, site selectivity and polarity of solvent used [14] [15] [16] .
Based upon these observations, the present work tends to use various routes to synthesise 6-substituted-3-thioxo-1,2,4-triazin-5-one derivatives as Sencore analogue (Figure 1) [14] , and evaluate their antimicrobial activity in compare with Mycostatine and pipiricillin as standard antibiotic.
2. Results and Discussion
2.1. Chemistry
In order to synthesise targeted compounds, (E)-4-(4’-Bromophenyl)-2-oxo-3-buteneoic acid (1) used as starting material, which was prepared via treatment of 4-bromobenzaldehyde with sodium pyruvate in aq NaOH then ice bath [17] . Also dithioic formic hydrazide (2) synthesized by addition of CS2 to hydrazine hydrate in dioxan at room temperature with continuous stirring (m.p 130˚C) [18] . Thiocarbohydrazide produced from reflux CS2 with hydrazine hydrate in aqueous ethanol (m.p 170˚C) [19] .
Condensation of compound 1 with thiosemicarbazide by reflux in EtOH-NaOAc produced thiosemicarbazone 3 which upon boiling with K2CO3-EtOH afforded 6-(4-bromostyryl)-3-thioxo-3,4-dihydro-1,2,4-triazin-5(2H)one 4, (Scheme 1).
On the other hand, condensation of compound 1 with dithioic formic acid hydrazide (2) in reflux EtOH-AcOH yielded the hydrazine 5. A successful ring closure reaction of 5 occurred after reflux with sulfanilamide in DMF lead to the direct formation of 4-aryl-6-(4’-bromostyryl) -3-thioxo-3,4-dihydro-1,2,4-triazin-5(2H)one (6) (Scheme 1). Formation of 6 might be take place via a nucleophilic attack of amino-group of sulfa to SH of compound 5 followed by heterocyclisation (Scheme 2).
Most of 6-alkyl-4-amino-3-thioxo-1,2,4-triazin-5-ones and their alkylated systems are used as herbicides Sencore [14] . Thus, in hope to obtaining new biocidal compounds having high affects towards microbes, 4-amino-6-(4’-bromostyryl)-3-thioxo-1,2,4-triazin-5-one (8) synthesised via simple condensation of compound 1 with thiocarbohydrazide (1:1 by mole) in warm EtOH-AcOH to give the hydrazine 7 which subjected to ring closure reaction by reflux in absolute EtOH containing a few drops of Conc. HCl (1%, 1 mL) afforded the target 8 (Scheme 1). Both the obtained compounds 4, 6 & 8 have stable tautomeric structural formula, thione
thiol (C=S
C-SH).
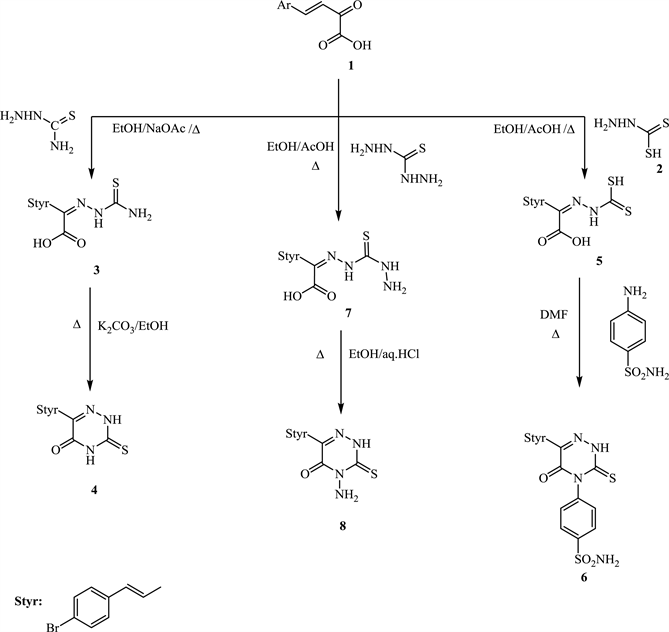
Scheme 1. Formation of 3-thioxo-1,2,4-triazin-5-one derivatives (4, 6 and 8).
UV absorption spectra gives us a good indication about their electronic distributions. λmax of both compounds 6 & 8 showed higher than that open structures 5 & 7. λmax of 6 (408), 5 (320), and 8 (425), 7 (420)nm. Also absorption bands of both 6 and 8 are slightly higher which might be due to extended conjugation of hetero-systems. IR spectra of all the obtained acyclic systems 3, 5 & 7 showed significant ν at 3500, 3200, 3100, 1610, 1200 and 750 cm−1 attribute the presence of OH, NH2, NH, HC=CH, C=S and C-Br fundamental groups, while that of cyclic compounds 4, 6 & 8 reduced a lack of OH groups, which confirm that heterocyclization reaction. 1HNMR spectrum of compound 4 recorded a resonated signals at δ 13.3, 11.5 for NH & NH of 1,2,4-triazine. In addition δ 8.0 - 7.3 ppm assigned aromatic and CH=CH protons with disappearance of both

Scheme 2. A plausible suggested mechanism via a nucleophilic attack of amino-group of sulfa to SH of compound 5 followed by heterocyclization.
OH and SH protons, while that of compound 8 showed at δ 7.8 - 7.7, 7.6 - 7.5 ppm (each double, 2H, aryl protons), 7.3-7.2 (coupling CH=CH-) 4.3 - 4.1 ppm for CH=CH- and 2.8, 2.3 ppm assigned NH2(N4) & NH(N2).
13C spectrum of compound showed a resonated signals at δ 180, 165, 150 & 142 ppm attribute C=S, C=O, HC=CH & C=N of 1,2,4-triazine nucleus, in addition δ at 130 - 120 ppm for aromatic carbons.
Mass fragmentation pattern of functionalized 1,2,4-triazines obtained recorded a lower intensity of that molecular ion with a base peak at 183, featured that 4-bromophenylethylene radical (Figure 2).
A higher stability of compound 8 as Sencore analogues, may be due to a various tautomeric and H-bonding via internal effects (Figure 3).
2.2. The Antimicrobial Evaluation
The newly prepared compounds 3 - 8 have been evaluated as antimicrobial agents against Gram-negative and Gram-positive bacteria as Escherichia Coli, Klebsiella pneumoniae, Pseudomonas aeruginosa and Staphylococcus Aureus,
![]()
Figure 2. A predicted Mass fragmentation pattern.
![]()
Figure 3. The tautomeric effects and H-bonding of 8.
were recovered on Nutrient agar and MacConkey agar. Two fungi, Candida albicans and Aspergillus fumigatus were isolated on Sabouraud Dextrose Agar (Oxide). The in vitro tested use agar diffusion disk method [20] . DMSO used as solvent and the antimicrobial potentialities of the tested compounds were estimated by placing pastoralized filter paper disks (11 mm diameter) impregnated with 50 mg/disk, and showed inhibition zones (IZ) measured after 24 - 28 h incubation at 37˚C for bacteria and after 5 days incubation at 28˚C for fungi. References antibiotic disks (11 mm in diameter) supplied by (BBL), piperacillin (PIP) and fungicide Mycostatine were used for comparison [21] . The results obtained outline in Table 1.
From the results obtained in Table 1. We can conclude that: Generally, the compounds 4, 6 and 8 exhibit a growth inhibition activity more than open derivatives. The compound numbered 8 recorded the highest inhibition activity more than other compounds including the standard antibiotic used. The Structure Activity Relationship (SAR) showed that presence of a sulfonamide, mercapto and amino groups led to enhance the activity. Minimum inhibitory concentration (MIC) for the active synthesised compounds as 4, 6 and 8 displayed a very good antimicrobial activity in compared with the standard antibiotic used.
3. Experimental
The melting point recorded on Stuart scientific SMP3 (Bibby, UK) melting point apparatus and reported as uncorrected. A Perkin Elmer (Lambda EZ-2101) double beam spectrophotometer (190 - 1100 nm) used for recording the electronic spectra. A Perkin Elmer model RXI-FT-IR 55,529 cm−1 used for recording the IR spectra. A Brucker advance DPX 400 MHz using TMS as an internal standard for recording the 1H NMR 13C nmr spectra in deuterated DMSO (δ in ppm). AGC-MS-QP 1000 Ex model used for recording the mass spectra. Elemental analysis performed on Micro Analytical Center of National Reaches Center-Dokki, Cairo, Egypt.
![]()
Table 1. The Antimicrobial evaluation of the new compounds 3 - 8.
A: E. coli, B: K. pneumonia, C: P. aeruginosa, D: S. coccus, E: C. albicans, F: A. fumigates, P: Pipericillin, M: Mycostatine.
・ (2Z,3E)-4-(4-bromophenyl)-2-(2-carbamothioylhydrazineylidene)but-3-enoic acid (3)
Thiocarbohydrazide obtained from reflux hydrazine hydrate with CS2 in aq EtOH for 30 min. then cooled and filtered to give the solid (m.p 170˚C). A mixture of 1 (0.01 mol) in AcOH (50.0 ml) and thiosemicarbazide (0.01 mol) in hot water (10.0 ml) reflux for 1 h, cooled then powered onto ice. The solid obtained was filtered off and crystallized from MeOH to give 3. Yield 80%, m.p 209˚C - 210˚C. IR νmax cm−1: 3500 - 3300 (OH, NH2), 3120 (NH), 1700 (C=O), 1600 (CH=CH), 1190 (C-S), 750 (C-Br). 1HNMR (400 MHz, DMSO-d6) δH ppm: 8 - 7.8 (d, 1H, Ar H), 7.56 (d, 1H, ArH), 7.35 (s, 1H, ArH), 7.25 (s, 1H, ArH), 7.2 - 6.9 (d, d, CH=CH), 5.49 (s, 1H, OH), 4.2 (s, 1H, NHCS), 3.3 (s, 2H, NH2CS). Analytical data for C11H10N3SO2Br (328); Calculated C 40.24; H 3.04; N, 12.80; S 9.75%, Found: C 40.01; H, 3.00; N 12.55; S 9.49%.
・ 6-(4-bromostyryl)-3-thioxo-3,4-dihydro-1,2,4-triazin-5(2H)-one (4)
Compound 3 (1.0 gm) in ethanol (100.0 ml) with K2CO3 (5.0 gm) reflux for 4 h, cooled then powered onto ice-HCl. The resultant solid filtered off and recrystallized from ethanol to give 4. Yield 65%, m.p. 190˚C - 192˚C. UV (λ) nm: 375. IR (ν)cm−1: 3180, 3150 (2NH), 1670 (C=O), 1620 (CH=CH-), 1580 (C=N), 1350 (cyclic NCSN), 1180 (C-S). 1HNMR (400 MHz, DMSO-d6) δH ppm: 13.3, 11.5 (each s, NH, NH), 8.0 - 7.3 (d, 1H, ArH), 7.56 (d, 1H, Ar-H), 7.35 (s, 1H, ArH), 7.2 - 6.9 (d,d, CH=CH), coupling CH=CH), 7.1 - 6.9 (d, d, 4-substituted phenyl), 4.3 - 4.1 (m, CH=CH),. Analytical data, for C11H8N3SOBr (310); Calculated C 42.58; H 2.58; N 13.54; S 10.32%, Found: C 42.19; H 2.30; N 13.11; S 10.00%.
・ (2Z,3E)-4-(4-bromophenyl)-2-(2-dithiocarboxyhydrazineylidene)but-3-enoic acid (5)
A mixture of 1 (0.01 mol) and dithioic formic acid hydrazide 2 (0.01 mol) in AcOH-EtOH (1:1, 50 ml) reflux for 1h, cooled then poured onto ice. The resultant solid filtered off and crystallized from EtOH to give 5. Yield 70%, m.p. 193˚C - 195˚C. IR (ν)cm−1: 3480 (OH), 3180 (NH), 1610 (CH=CH-), 1710 (C=O), 1570 (C=N), 1180 (C-S), 710 (C-Br).1HNMR (400 MHz, DMSO-d6) δH ppm: 7.75 (d, 1H, ArH), 7.40 (d, 1H, ArH), 7.25 (s, 1H, ArH), 7.25 - 6.8 (d, d, CH=CH), 5.78 (s, 1H, SH), 5.41 (s, 1H, OH), 4.1 (s, 1H, NHCS). Analytical data, for C11H9N2S2O2Br (345); Calculated C 38.26; H 2.60; N 8.11; S 18.55%, Found: C 38.15; H 2.35; N 8.01; S 18.30%.
・ 4-(6-(4-bromostyryl)-5-oxo-3-thioxo-2,5-dihydro-1,2,4-triazin-4(3H)-yl)benzenesulfonamide (6)
Equimolar amounts of 5 and sulfanilamide in DMF (100.0 mL), reflux for 6h, cooled then poured onto ice. The yielded solid filtered off and crystallized from Methanol to give 6. Yield 66%, m.p. 178˚C - 180˚C. UV (λ) nm: 408. IR (ν) cm−1: 3080 (NH), 1690 (C=O), 1610 (CH=CH-), 1350, 1280 (SO2-NH2) 1188 (C-S), 910, 830 (p-substituted phenyl). 1HNMR (400 MHz, DMSO-d6) δH ppm: 11.8 (s, 1H, NH), 8.0 - 7.8 (d, d, p-substituted phenyl), 7.2 - 7.0 (m, 2H, coupling CH=CH) 6.9 - 6.6 (m, 4H, aromatic), 3.4 (s, 2H, NH2). Analytical data, for C16H13N4S2O3Br (451); Calculated. C 43.77; H 3.00; N 12.01; S 13.73%, Found: C 43.55; H 2.88; N 11.70; S 13.51%.
・ (2Z,3E)-4-(4-bromophenyl)-2-(2-(hydrazinecarbonothioyl)hydrazineylidene)but-3-enoic acid (7)
A mixture of 1 (0.01 mol) and thiocarbohydrazide (0.01 mol) in AcOH-EtOH (1:1, 100 mL) reflux for h, cooled then poured onto ice. The solid that obtained filtered off and crystallised from EtOH to give 7. Yield 65%, m.p. 155˚C - 157˚C. UV (λ) nm: 420. IR (ν) cm−1: 3500 - 3400 (OH, NH2), 3300, 3180 (NH, NH), 1700 (C=O), 1610(CH=CH-), 1190 (C=S), 880 (p-substituted phenyl) 710 (C-Br). 1HNMR (400 MHz, DMSO-d6) δH ppm: 7.81 (d, 1H, ArH), 7.55 (d, 1H, ArH), 7.35 (s, 1H, ArH), 7.2 - 6.9 (d,d, CH=CH), 4.11 (s, 1H, NHCS), 3.8 (s,1H, NH-NH2), 3.30 (s, 2H, NH2). Analytical data, for C11H9N4SOBr (325); Calculated C 40.61; H 2.76; N 17.23; S 9.84%, Found: C 38.02; H 3.01; N 16.12; S 9.25%.
・ 4-Amino-6-(4-bromostyryl)-3-thioxo-3,4-dihydro-1,2,4-triazine-5(2H)-one (8)
Compound 7 (1.0 gm) in aq. HCl (1%, 100 mL), ethanol (100.0 mL), reflux for 2 h, cooled then neutralized with NaHCO3. The produced solid filtered off and crystallized from MeOH, to give 8. Yield 50%, m.p. 176˚C - 178˚C. UV (λ) nm: 425. IR (ν) cm−1: 3300 - 3180 (NH2, NH), 1700 (C=O), 1640 (deformation NH2), 1610 (HC=CH), 1480 (CH=CH), 1188 (C=S), 890 (p-substituted phenyl) 720 (C-Br).). 1HNMR (400 MHz, DMSO-d6) δH ppm: 12.0 (s, 1H, NH), 7.8 - 7.7, 7.6 - 7.5 (m, 2H, aromatic protons), 7.3 - 7.2 (coupling -CH=CH-), 4.3 - 4.1 (m, -CH=CH-), 2.88 (s, 2H, NH2). 13C nmr spectrum (DMSO-d6) δC ppm: 180 (C=S), 165 (C=O), 150 (C=C), 142 (C=N), 130 - 120 (aromatic carbons). Analytical data, for C11H9N4SOBr (325); Calculated C 40.61; H 2.76; N 17.23; S 9.84%, Found: C 40.15; H 2.55; N 17.01; S 9.59%,
4. Conclusion
Various routes to synthetic polyfunctional 1,2,4-triazine derivatives have been used (E)4-aryl-2-oxo-3-buteneoic acid (1), dithioic formic acid hydrazide, thiosemicarbazide and thiocarbohydrazide in different media. Most of these targets exhibit a very good antibacterial and antifungal activity in compared with the reference antibiotics used.