New Method for Preparation of 1-Amidoalkyl-2-Naphthols via Multicomponent Condensation Reaction Utilizing Tetrachlorosilane under Solvent Free Conditions ()
1. Introduction
A recent increased attention witnessed to the use of tetrachlorosilane (TCS), a cheap industrial intermediate, in different areas of the organic chemistry has now reached significant levels, not only for the possibility to perform environmentally benign synthesis, but for the good yield. In continuation of our investigations on the development and applications of new in situ reagents derived from tetrachlorosilane in organic synthesis [1] - [9] , we have developed an efficient, general, and convenient protocol for the multicomponent synthesis of 1-ami- doal-kyl-2-naphthols.
Multicomponent coupling reactions (MCRs) represent powerful time-, energy-, and material-saving synthetic protocols in modern chemistry in which molecular complexity could be generated in a single synthetic operation [10] [11] .
The preparation of 1-amidoalkyl-2-naphthols can be carried out by multi-component condensation of aryl aldehydes, β-naphthol and amide derivatives in the presence of Lewis or Bronsted acid catalysts such as Ce(SO4)2 [12] , montmorillonite K10 [13] , iodine [14] , cation exchanged resins [15] , NaHSO4.H2O [16] , Fe(HSO4)3 [17] , sulfamic acid/ultrasound [18] , HClO4/SiO2 [19] , cyanuric chloride [20] and K5CoW12O40∙3H2O [21] . Some of the reported methods suffer from one or more limitations such as high reaction temperature, lower product yield, tedious work up, and use of toxic reagents. They also lack general applicability to produce arrays of 1-ami- doalkyl-2-naphthols as they are restricted to only a few amides. Therefore, the development of a more general, cost-effective multicomponent coupling reaction (MCR) protocol for the synthesis of 1-amidoalkyl-2-naphthols remains a challenge. Therefore the introduction of new, efficient, and general methods involving various nitriles for this multicomponent reaction under milder conditions is still required.
2. Experimental
Infrared (IR) spectra were recorded on JASCO 410 spectrometer. Absorption maxima were recorded in cm-1. Nuclear Magnetic Resonance (NMR) spectra were run at Varian-Mercury (300 MHz) FTNMR spectrometer. Spectra were taken using DMSO solvent with chemical shifts quoted in parts per million (δ ppm) using TMS as internal standard. The Mass Spectra (M.S.) were recorded on GC-MS QP-2010 EX Schmiadzu (Japan) mass spectrometer. Melting points (uncorrected) were determined in an open capillary with a Griffin melting point apparatus. Column Chromatography was carried out by using Merck Kieselgel 60 GF-254 (230 - 400 mesh). Analytical TLC was performed on aluminum sheets (Merck, Kieselgel 60 F254, Thickness 0.2 mm). Tetrachlorosilane (TCS) was used as obtained from commercial sources. The solvents were distilled and dried before use. Acetonitrile was dried by refluxing over phosphorous pentoxide then distilled. Methylene chloride was dried by refluxing over anhydrous calcium chloride then distilled.
Typical Procedure
In 50 ml round bottom flask equipped with air condenser and magnetic stirring bar, a mixture of aromatic aldehyde (5 mmol), anhydrous naphthol (5 mmol), and tetrachlorosilane (1.8 ml, 15 mmol) was allowed to stir at room temperature for 10 minutes. To this reaction mixture, 5 mmol of nitrile [acetonitrile or benzonitrile] was added and the stirring process was continued for further time (monitored by TLC). The reaction mixture was quenched using ice cold water. The aqueous solution was extracted with chloroform (3 × 30 ml) and then the chloroform extract was dried over anhydrous Na2SO4 and concentrated over boiling water bath. The residual oil was purified using preparative thin layer chromatography using silica gel to give the products in pure form.
(1) N-((2-Hydroxynaphthalen-1-yl)(phenyl)methyl)acetamide:
mp 246˚C (lit. [12] mp 241˚C - 243˚C); IR (KBr, cm−1): 3399, 3245, 3062, 1639, 1581, 1517, 1369, 1334, 1101, 806, 740, 692, 617; 1H-NMR(300 MHz, DMSO-d6): δ (ppm) 1.97 (s, 3H), 7.1 - 7.35 (m, 9H),7.74 - 7.85 (m, 3H), 8.38 (d, J = 9 Hz, 1H), 9.95 (s, 1H); 13C NMR(75 MHz, DMSO-d6): δ (ppm) 23.5, 41.3, 118.8, 120.2, 122.0, 123.9, 124.9, 125.7, 127.6, 128.1, 128.3, 128.5, 128.6, 134.2, 144.0, 152.6, 169.6; MS: m/z 231(100%), 232(75.7%), 233(13.16%), 291(20.72%)M+.
(2) N-((4-chlorophenyl)(2-hydroxynaphthalen-1-yl)methyl)acetamide
mp 230˚C (lit. [12] mp 224˚C - 227˚C); IR (KBr, cm−1): 3394, 2960, 2705, 2609, 1631, 1581, 1521, 1436, 1371, 1328, 1272, 1240, 1170, 1091, 815, 748, 586, 499.
(3) N-((2-hydroxynaphthalen-1-yl)(p-tolyl)methyl)acetamide:
mp 224˚C (lit. [17] mp 222˚C - 223˚C); IR (KBr, cm−1): 3394, 2969, 2707, 2616, 1625, 1515, 1436, 1332, 1272, 1174, 1064, 811; 1H-NMR(300 MHz, DMSO-d6): δ (ppm) 2.02 (s, 3H), 2.24 (s, 3H), 7.05 - 7.40(m, 8H), 7.76 - 7.94 (m, 3H), 8.45 (d, J = 8.7 Hz, 1H), 10.25 (s, 1H); 13C NMR(75 MHz, DMSO-d6): 20.50, 22.68, 47.74, 118.53, 119.04, 122.33, 123.31, 126.03, 126.23, 128.52, 129.11, 132.35, 135.04, 139.56, 153.13, 169.18; MS: m/z 245(100%), 246(75.7%), 247(13.1%), 305(21%)M+.
(4)N-((4-(dimethylamino)phenyl)(2-hydroxynaphthalen-1-yl)methyl)acetamide:
mp 215˚C (lit. [12] mp 215˚C - 217˚C); IR (KBr, cm−1): 3432, 3021, 2886, 2803, 1664, 1523, 1469, 748.
(5) N-((4-formylphenyl)(2-hydroxynaphthalen-1-yl)methyl)acetamide:
mp 232˚C (lit. [22] mp 237˚C - 240˚C); IR (KBr, cm−1): 3386, 3056, 1700, 1627, 1604, 1272, 817.
(6) N-((2-bromophenyl)(2-hydroxynaphthalen-1-yl)methyl)acetamide:
mp 210˚C (lit. [23] mp 204˚C); IR (KBr, cm−1): 3421, 3122, 1656, 1517, 1434, 1058, 817, 752.
(7) N-((3-bromophenyl)(2-hydroxynaphthalen-1-yl)methyl)acetamide:
mp 230˚C (lit. [24] mp 229˚C - 230˚C); IR (KBr, cm−1): 3407, 3164, 1643, 1517, 1436, 1066, 811, 748.
(8) N-((2-nitrophenyl)(2-hydroxynaphthalen-1-yl)methyl)acetamide:
mp 215˚C (lit. [25] mp 218˚C - 219˚C); IR (KBr, cm−1): 3410, 3265, 1643, 1590, 1520, 1415, 1345, 1305, 1225, 1105, 1010, 950, 852, 745.
(9) N-((3-nitrophenyl)(2-hydroxynaphthalen-1-yl)methyl)acetamide:
mp 180˚C (lit. [25] mp 256˚C - 258˚C); IR (KBr, cm−1): 3241, 1625, 1596, 1525, 1436, 1346, 1286, 1211, 1091, 1014, 948, 856, 732; 1H-NMR(300 MHz, DMSO-d6): δ (ppm) 2.06 (s, 3H), 7.15 - 7.49 (m, 6H), 7.78 - 8.04 (m, 5H), 8.54 (d, J = 8.1 Hz, 1H), 10.12 (s, 1H); 13C NMR(75 MHz, DMSO-d6): 25.55, 66.13, 108.61, 118.16, 120.28, 122.32, 123.94, 125.57, 127.46, 128.4, 129.20, 129.46, 130.84, 132.08, 134.82, 147.69, 148.04, 152.49, 191.67; MS: m/z 276(100%), 277(75.7%), 278(13.16%), 336(13%)M+.
(10) N-((4-methoxyphenyl)(2-hydroxynaphthalen-1-yl)methyl)acetamide:
mp 178˚C (lit. [17] mp 183˚C - 186˚C); IR (KBr, cm−1): 3399, 3245, 1639, 1511, 1438, 1365, 1303, 1247, 1172, 1029, 815, 748.
(11) N-((2-hydroxynaphthalen-1-yl)(phenyl)methyl)benzamide:
mp 230˚C (lit. [26] mp 235˚C - 237˚C); IR (KBr, cm−1): 3413, 3222, 1629, 1531, 1488, 1346, 752; 1H-NMR (300 MHz, DMSO-d6): δ (ppm) 7.23 - 8 (m, 17H), 9.01 (1H, d, J=9 Hz), 10.3 (1H, s); 13C NMR(75 MHz, DMSO-d6): 167.30, 155.13, 142.59, 135.39, 132.59, 131.44, 130.27, 128.53, 128.42, 128.08, 127.98, 127.36, 126.99, 126.88, 126.56, 122.55, 117.26, 62.66; MS: m/z 233 (6.5%), 232 (42.25%), 231 (100%), 353 (4.74%) M+.
(12) N-((4-chlorophenyl)(2-hydroxynaphthalen-1-yl)methyl)benzamide:
mp 191˚C (lit. [27] mp 185˚C - 186˚C); IR (KBr, cm−1): 3424, 3116, 1633, 1569, 1535, 1484, 1434, 1344, 1270, 819, 707.
(13) N-((2-hydroxynaphthalen-1-yl)(p-tolyl)methyl)benzamide:
mp 213˚C (lit. [27] mp 216˚C - 218˚C); IR (KBr, cm−1): 3413, 1631, 1533, 1346, 1272, 1078, 817.
(14) N-((2-hydroxynaphthalen-1-yl)(4-methoxyphenyl)methyl)benzamide:
mp 196˚C (lit. [28] mp 197˚C - 199˚C); IR (KBr, cm−1): 3423, 1625, 1571, 1511, 1436, 1348, 1263, 1174, 1029, 933, 821; 1H-NMR (300 MHz, DMSO-d6): δ (ppm) 10.38 (1H, s), 9.05 (1H, d, J = 8.7 Hz), 8.11 (1H, d, J = 8.4 Hz),7.79 - 7.90 (3H,m), 7.45 - 7.55 (2H,m),7.25 - 7.32 (3H,m), 6.84 (2H, d, J = 8.7 Hz), 3.68 (3H, s); 13C NMR(75 MHz, DMSO-d6): 48.98, 54.98, 113.61, 118.48, 118.76, 122.65, 126.69, 127.02, 127.73, 128.38, 128.47, 12858, 129.21, 131.33, 132.25, 133.92, 134.43, 153.07, 158.05, 165.54; MS: m/z 263 (16.5%), 262 (40.5%), 261 (100%), 383 (5.7%) M+.
(15) N-((4-(dimethylamino)phenyl)(2-hydroxynaphthalen-1yl)methyl)benzamide:
mp 220˚C (lit. [25] mp 220˚C - 221˚C); IR (KBr, cm−1): 3405, 3273, 1631, 1573, 1521, 1434, 1340, 1180, 1029, 939, 811.
(16) N-((2-hydroxynaphthalen-1-yl)(2-nitrophenyl)methyl)benzamide:
mp 261˚C (lit. [25] mp 266˚C - 267˚C); IR (KBr, cm−1): 3440, 3145, 1649, 1510, 1490, 1441, 1347, 1249, 805, 712.
(17) N-((2-hydroxynaphthalen-1-yl)(3-nitrophenyl)methyl)benzamide:
mp 220˚C (lit. [21] mp 216˚C - 218˚C); IR (KBr, cm−1): 3413, 3180, 1644, 1522, 1468, 1428, 1356, 1272, 800, 712.
(18) N-((2-hydroxynaphthalen-1-yl)(4-nitrophenyl)methyl)benzamide:
mp 226˚C (lit. [21] mp 228˚C - 230˚C); IR (KBr, cm−1): 3444, 3155, 1649, 1510, 1493, 1447, 1347, 1244, 803, 713; 1H-NMR(300 MHz, DMSO-d6): δ (ppm) 10.24 (s, 1H, OH), 7.98 (d, J =7.8 Hz, 1H), 9.00 (d, J = 8.0 Hz, 1H), 7.10 - 7.25 (m, 4H, ArH), 7.46 - 7.66 (m, 7H, ArH), 7.76 - 7.98 (m, 4H, ArH); 13C NMR(75 MHz, DMSO- d6): 48.20, 116.29, 118.78, 119.94, 122.49, 125.42, 126.91, 128.54, 128.84, 129.55, 130.27, 131.48, 131.96, 132.69, 134.32, 141.78, 153.72, 165.44; MS: m/z 278(4.5%), 277(43.25%), 276(100%), 384(4.%) M+.
3. Results and Discussion
With the aim to develop more efficient synthetic processes and convenient protocol; for the one-pot synthesis of 1-amidoalkyl-2-naphthols, biologically active drug like molecules [29] - [31] . We herein describe a practical, inexpensive protocol for the preparation of 1-amidoalkyl-2-naphthols via multi component condensation reaction between various aromatic aldehydes, β-naphthols and nitriles including alkyl and aryl nitrile using readily available tetrachlorosilane (TCS) reagent at room temperature, and solvent-free conditions. To test the general scope and versatility of this procedure in the synthesis of a variety of substituted amidoalkyl naphthols, we examined a number of different substituted aromatic aldehydes, 2-naphthol and acetonitrile. To optimize the reaction condition, the reaction of benzaldehyde, β-naphthol and acetonitrile was selected as a model to investigate the effects of different amounts of reagent on the yield. The best result was obtained by carrying out the reaction with one molar amounts of aldehyde and β-naphthol, and two molar amounts of acetonitrile as shown in Table 1. We also examined the reaction in various solvents. Chlorinated solvents such as methylene chloride or 1,2-dichloroethane were found to be ineffective solvents yielding the reaction product in very low conversion. The donor solvents such as tetrahydrofuran (THF) and diethyl ether (DEE) were completely inhibited the reaction.
The reaction between benzaldehyde, acetonitrile and 2-naphthol was carried out without TCS and we found that no reaction takes place. To determine the optimum quantity of TCS, the reaction was carried out at room temperature. The use of two equimolar amounts of TCS, resulted in the highest yield. The molar ratio of aldehyde, β-naphthol, acetonitrile and TCS was kept at 1:1:2:2, respectively (Scheme 1).
Thus we prepared a range of 1-amidoalkyl-2-naphthols under the optimized reaction conditions: stirring the β-naphthol (1 mmol), aryl aldehydes (1 mmol) and acetonitrile (2 mmol) in the presence of tetrachlorosilane (2 mmol) at room temperature. A series of 1-amidoalkyl-2-naphthols were prepared in high to excellent yields (Table 2).
In the case of aromatic aldehydes the three-component reaction proceeded smoothly to give the corresponding 1-amidoalkyl-2-naphthols in high yields. Due to the availability of a vast number of aromatic aldehydes, this three component reaction can be very useful to synthesis the desired products.
As Table 2 shows that, the reaction proved to be general and tolerated a variety of aromatic aldehydes with substituent carrying either electron-donating (Table 2, entries 2, 3, 4, 6, 7, and 10) or electron-withdrawing groups (Table 2, entries 5, 8, and 9). Although as can be seen from the results of table, this reaction is affected by electronic and steric factors.
The suggested mechanism for this reaction is depicted in Scheme 2. This mechanism involves 1,2-addition of tetrachlorosilane (TCS) to aldehyde to produce silyl ether intermediate [A]. On the other hand, 2-naphthol reacted with TCS and produce silyl enol ether [B] and HCl. As reported in literature [32] [33] the reaction of 2-naphthol with aromatic aldehydes in the presence of Lewis acid is known to give ortho-quinone methides (o-QMs). The same o-QMs, generated in-situ, intermediate [E] have been reacted with nitrilium salt [34] [35] [C] to form 1-amidoalkyl-2-naphthol after hydrolysis (Scheme 1). The reaction gave the desired products in the Ritter type reaction [36] [37] at room temperature.
To our knowledge, there is no general protocol employing aromatic nitrile in such addition so far. Therefore, we examined the reaction of benzonitrile with aldehyde, β-naphthol and TCS as representative example to aryl nitrile. It is noteworthy that no reaction was observed under the above conditions to give the corresponding

Scheme 1. Reaction of different aldehydes, acetonitrile and 2-naphthol.
![]()
Table 1. Effect of different molar ratio on the yield of product.
![]()
Table 2. Reaction of aldehydes with β-naphthol and acetonitrile in the presence of tetrachlorosilane.
1-amidoalkyl-2-naphthols typically. The use of two equivalents of benzonitrile and tetrachlorosilane and one equivalent of zinc chloride as a binary reagent in the reaction resulted in the highest yield of N-((2-hydrox- ynaphthalen-1-yl) (phenyl)methyl)benzamide, which might be attributed to steric factors as well as to the low of nucleophilicity of benzonitrile (Scheme 3).
As shown in Table 3, reaction of aldehydes with β-naphthol and benzonitrile in the presence of tetrachlorosilane and zinc chloride as a binary reagent under solvent- free conditions at room temperature produced highest yields from 1-benzamidomethyl-2-naphthol derivatives.
Attempts to bring aliphatic aldehydes such as acetaldehyde into the reaction with 2-naphthol and acetonitrile under mild conditions were mostly unsuccessful. Therefore no other aliphatic Aldehydes were examined. To regard the purity of the compounds prepared, melting point and infrared spectra were matched with previously reported literature data. NMR and MS analysis to some compounds revealed the correct structure of the products obtained.
4. Conclusion
An efficient one-pot method has been developed for the synthesis of 1-amidoalkyl-2-naphthols from condensation of aldehyde, β-naphthol, acetonitrile and TCS as condensing reagent. The present methodology gives several
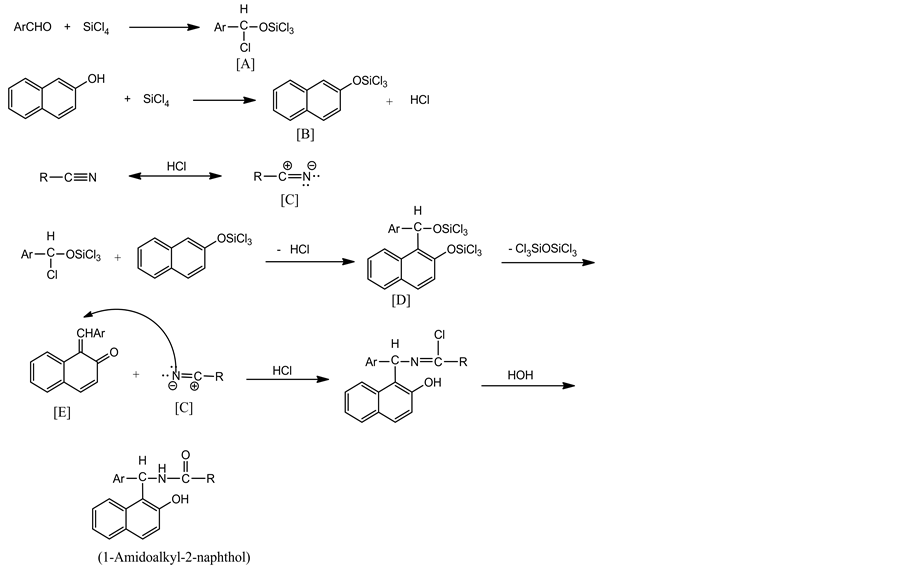
Scheme 2. Suggested mechanism for the formation of 1-amidoal-kyl-2-naph- thol.

Scheme 3. Reaction of different aldehydes, benzonitrile and 2-naphthol.
![]()
Table 3. Synthesis 1-benzamidomethyl-2-naphthol derivatives in the presence of TCS/ZnCl2 at r.t. under solvent- free conditions.
advantages such as simple procedure, easy workup, high yields, and solvent free reaction conditions. The salient feature of this methodology includes an easy purification, generality and in addition no cumbersome apparatus were needed.
NOTES
*Corresponding author.