1. Introduction
Dengue is a vector borne disease transmitted to humans by the bite of an infected female Aedes mosquito [1] . Dengue fever (DF) also known as break-born fever is a mosquito born infection that causes a severe flu-like illness, caused by any one of the four closed related dengue viruses transmitted by female mosquitoes, i.e. DEN-1, DEN-2, DEN-3 and DEN-4. The first recognized Dengue epidemics occurred almost simultaneously in Asia, Africa, and North America in the 1780s, shortly after the identification and naming of the disease in 1779. It has spread especially in the tropical and sub tropical regions around the world, and nowadays is a disease widely found in urban and semi-urban areas, ([2] ). Mathematical modelling is the interesting tool for understanding epidemiological diseases and for proposing effective strategies to fight them ([3] ). The mathematical model of dengue transmission is a multi-population model that captures the transmission dynamics between host (human) and vector (mosquito) taking into account the four strains of dengue virus and the cross infections. Various models have been proposed to study factors on the transmission dynamics and control the spread of dengue fever disease ([2] -[10] , studied a dengue model by evaluating and analysing the sensitivity indices of the reproduction number 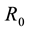 in order to determine the relative importance of the model parameters in the disease transmission. So far no one considered a dynamical system that incorporates the effects of treatment in dengue fever disease model. In this paper, it is therefore intended to analyse a model which will include treatment. Thus, we study and analyse a non linear mathematical model showing the effect of treatment on the transmission of dengue fever disease in the population.
in order to determine the relative importance of the model parameters in the disease transmission. So far no one considered a dynamical system that incorporates the effects of treatment in dengue fever disease model. In this paper, it is therefore intended to analyse a model which will include treatment. Thus, we study and analyse a non linear mathematical model showing the effect of treatment on the transmission of dengue fever disease in the population.
2. Model Formulation
A non linear mathematical model is formulated and analysed showing the effect of treatment of Dengue fever disease. The basic reproduction number and stability of equilibrium points are analysed qualitatively. Sensitivity analysis of parameters and numerical simulations are performed. The total human population at any time t will be denoted by 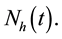 The total population is subdivided into four sub-populations namely; Susceptibles
The total population is subdivided into four sub-populations namely; Susceptibles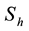 , Infectives
, Infectives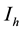 , Treated
, Treated 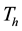 and Resistant
and Resistant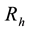 .
.
Thus
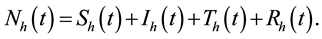
where ―represent human population.
―represent human population.
There are three other state variables, related to the female mosquitoes, indexed by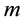 :
:
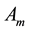 ―Aquatic phase (that includes the egg, larva and pupa stages);
―Aquatic phase (that includes the egg, larva and pupa stages);
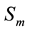 ―Susceptibles (mosquitoes that are able to contract the disease);
―Susceptibles (mosquitoes that are able to contract the disease);
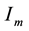 ―Infectives (mosquitoes capable of transmitting the disease to human).
―Infectives (mosquitoes capable of transmitting the disease to human).
In formulating the model, the following assumptions are considered:
i) Total human population 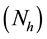 is constant at any time t, i.e.
is constant at any time t, i.e.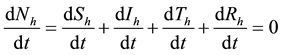 ,
,
ii) The population is homogeneous, which means that every individual of a compartment is homogeneously mixed with the other individuals,
iii) Immigration and emigration are not considered,
iv) Each vector has an equal probability to bite any host,
v) Humans and mosquitoes are assumed to be born susceptible i.e. there is no natural protection,
vi) The coefficient of transmission of the disease is fixed and does not vary seasonally,
vii) For the mosquito there is no resistant phase, due to its short lifetime, ([10] ).
Considering the above assumptions, we then have the following
Schematic model flow diagram for dengue fever disease with treatment:
From Figure 1 flow diagram, the model will be governed by the following equations:
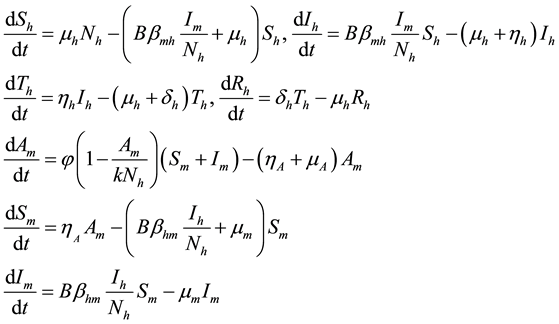 (1)
(1)
where
![]()
Figure 1. Model flow diagram for dengue fever disease with treatment.
![]() ,
, ![]() ,
, ![]() ,
, ![]() ,
, ![]() ,
, ![]() ,
, ![]() , for all
, for all![]() .
.
3. Model Analysis
The model system of Equation (1) will be analysed qualitatively to get a better understanding of the effects of treatment of Dengue fever disease. The basic Reproduction number ![]() which governs elimination or persistence of Dengue fever disease will be determined and studied.
which governs elimination or persistence of Dengue fever disease will be determined and studied.
3.1. Disease Free Equilibrium (DFE)
For the disease free equilibrium, it is assumed that there is no infection for both populations of human and mosquitoes i.e. ![]() and
and![]() , denoted by “
, denoted by “![]() ”. Thus
”. Thus ![]() of the model system (1) is obtained as
of the model system (1) is obtained as
![]() (2)
(2)
3.2. The Basic Reproduction Number, “R0”
The basic reproduction number, denoted by![]() , is defined as the average number of secondary infections that occurs when one infective individual is introduced into a completely susceptible population ([11] ).
, is defined as the average number of secondary infections that occurs when one infective individual is introduced into a completely susceptible population ([11] ).
The basic reproduction number of the model (1) ![]() is calculated by using the next generation matrix of an ODE ([11] ). Using the approach of ([11] ).
is calculated by using the next generation matrix of an ODE ([11] ). Using the approach of ([11] ). ![]() is obtaining by taking the largest (dominant) eigenvalue (spectral radius) of
is obtaining by taking the largest (dominant) eigenvalue (spectral radius) of
![]()
where, ![]() is the rate of appearance of new infection in compartment
is the rate of appearance of new infection in compartment![]() ,
, ![]() is the transfer of individuals out of the compartment
is the transfer of individuals out of the compartment ![]() by all other means and
by all other means and ![]() is the disease free equilibrium.
is the disease free equilibrium.
![]()
Using the linearization method, the associated matrix at DFE is given by
![]()
This implies that
![]()
With![]() ,
, ![]() we have
we have
![]()
or
![]()
The transfer of individuals out of the compartment ![]() is given by
is given by
![]()
Using the linearization method, the associated matrix at DFE is given by
![]()
This gives ![]() with
with
![]()
Therefore
![]() (3)
(3)
The eigenvalues of the Equation (3) are given by
![]()
This gives
![]()
![]()
It follows that the Reproductive number which is given by the largest eigenvalue for model system (1) with treatment denoted by ![]() is given by
is given by
![]() (4)
(4)
where![]() .
.
If![]() , the disease cannot invade the population and the infection will die out over a period of time, and also, if
, the disease cannot invade the population and the infection will die out over a period of time, and also, if![]() , then an invasion is possible and infection can spread through the population. Generally, the larger the value of
, then an invasion is possible and infection can spread through the population. Generally, the larger the value of![]() , the more severe, and possibly widespread the epidemic will be, ([10] ).
, the more severe, and possibly widespread the epidemic will be, ([10] ).
3.3. Sensitivity Analysis of Model Parameters
In order to determine how best human mortality due to dengue fever is reduced, we calculate the sensitivity indices of the reproduction number ![]() to each parameter in the model using the approach of ([11] ). These indices tell us which parameters have high impact on
to each parameter in the model using the approach of ([11] ). These indices tell us which parameters have high impact on ![]() and should be targeted by intervention strategies. Also Sensitivity indices allow us to measure the relative change in a variable when a parameter changes. The normalized forward sensitivity index of a variable with respect to a parameter is the ratio of the relative change in the variable to the relative change in the parameter. When the variable is a differentiable function of the parameter, the sensitivity index may be alternatively be defined using partial derivatives.
and should be targeted by intervention strategies. Also Sensitivity indices allow us to measure the relative change in a variable when a parameter changes. The normalized forward sensitivity index of a variable with respect to a parameter is the ratio of the relative change in the variable to the relative change in the parameter. When the variable is a differentiable function of the parameter, the sensitivity index may be alternatively be defined using partial derivatives.
Definition 1: The normalized forward sensitivity index of “![]() ”, that depends differentiable on a parameter “
”, that depends differentiable on a parameter “![]() ”, is defined as ([12] )
”, is defined as ([12] )
![]() (5)
(5)
As we have an explicit formula for ![]() in the Equation (5), we derive an analytical expression for the sensitivity of
in the Equation (5), we derive an analytical expression for the sensitivity of ![]() as
as ![]() to each of parameters involved in
to each of parameters involved in![]() . For example, using the set of estimated parameter values given as
. For example, using the set of estimated parameter values given as![]() ,
, ![]() ,
, ![]() ,
, ![]() ,
, ![]() ,
, ![]() ,
, ![]() ,
, ![]() ,
, ![]() ,
, ![]() the sensitivity indices of
the sensitivity indices of ![]() with respect to
with respect to ![]() and
and ![]() are given by
are given by![]() and
and ![]() respectively. Otherindices
respectively. Otherindices![]() ,
, ![]() ,
, ![]() ,
, ![]() ,
, ![]() ,
, ![]() ,
, ![]() and
and ![]() are obtained following the same method and tabulated as follows:
are obtained following the same method and tabulated as follows:
The parameters are ordered from most sensitive to the least.
Interpretation of Sensitivity Indices
From Table 1 generally it shows that the parameters![]() ,
, ![]() ,
, ![]() ,
, ![]() ,
, ![]() and
and ![]() each increases keeping other parameters constant they increase the value of
each increases keeping other parameters constant they increase the value of ![]() implying that they increase the endemicity of the disease as they have positive indices. While the parameters
implying that they increase the endemicity of the disease as they have positive indices. While the parameters![]() ,
, ![]() ,
, ![]() and
and ![]() when each increases while keeping the other parameters constant they decrease the value of
when each increases while keeping the other parameters constant they decrease the value of ![]() implying that they decrease the endemicity of the disease as they have negative indices.
implying that they decrease the endemicity of the disease as they have negative indices.
But individually, the most sensitive parameter is the average daily biting (per day)![]() , followed by maturation rate from larvae to adult (per day)
, followed by maturation rate from larvae to adult (per day)![]() , transmission probability from
, transmission probability from ![]() (per bite)
(per bite)![]() , number of larvae per human
, number of larvae per human![]() , transmission probability from
, transmission probability from ![]() (per bite)
(per bite)![]() , number of eggs at each deposit per capita (per day)
, number of eggs at each deposit per capita (per day)![]() , then average lifespan of humans (per day)
, then average lifespan of humans (per day)![]() , natural mortality of larvae (per day)
, natural mortality of larvae (per day)![]() , mean viremic period (per day)
, mean viremic period (per day)![]() , and finally the least sensitive parameter is the average lifespan of adult mosquitoes (per day)
, and finally the least sensitive parameter is the average lifespan of adult mosquitoes (per day)![]() .
.
3.4. Local Stability of Disease Free Equilibrium Point
To determine the local stability of the disease free equilibrium, the variation matrix ![]() of the model system (1) corresponding to the disease free
of the model system (1) corresponding to the disease free ![]() is obtained as
is obtained as
![]() (6)
(6)
where ![]()
Therefore the stability of the disease free equilibrium point can be clarified by studying the behaviour of ![]() in which for local stability of DFE we seek for its all eigenvalues to have negative real parts. It follows that, the characteristic function of the matrix (6) with
in which for local stability of DFE we seek for its all eigenvalues to have negative real parts. It follows that, the characteristic function of the matrix (6) with ![]() being the eigenvalues of the Jacobian matrix, by using Mathe- matica software the Jacobian matrix has the following eigenvalues:
being the eigenvalues of the Jacobian matrix, by using Mathe- matica software the Jacobian matrix has the following eigenvalues:
![]()
Table 1. Sensitivity Indices of model parameters to![]() .
.
![]()
![]()
when ![]() is not a real number.
is not a real number.
The other eigenvalues are given as
![]()
![]()
![]()
when ![]() is not a real number,
is not a real number,
![]()
when ![]() is not a real number, and finally
is not a real number, and finally
![]()
when ![]() is not a real number.
is not a real number.
Hence under certain conditions the system is stable since all the seven eigenvalues are negative. These imply that at ![]() the Disease Free Equilibrium point is locally asymptotically stable, i.e. Dengue infection can be eliminated from the population.
the Disease Free Equilibrium point is locally asymptotically stable, i.e. Dengue infection can be eliminated from the population.
3.5. Global Stability of Disease Free Equilibrium Point
In this subsection, we analyse the global behaviour of the equilibria for system (1). The following theorem provides the global property of the disease free equilibrium ![]() of the system. The results are obtained by means of Lyapunov function. In choosing the Lyapunov function the idea of [13] is used.
of the system. The results are obtained by means of Lyapunov function. In choosing the Lyapunov function the idea of [13] is used.
Theorem 1: If![]() , then the infection-free equilibrium is globally asymptotically stable in the interior of
, then the infection-free equilibrium is globally asymptotically stable in the interior of![]() .
.
Proof:
To establish the global stability of the disease-free equilibrium, we construct the following Lyapunov function:
![]() (7)
(7)
Calculating the time derivative of ![]() along (7), we obtain
along (7), we obtain
![]()
Then substituting ![]() and
and ![]() from system (1), we get
from system (1), we get
![]()
where
![]()
It follows that
![]()
or
![]()
which is equivalent to
![]()
![]()
But ![]()
or
![]() (9)
(9)
Substituting (9) into (8) yields
![]()
Therefore
![]()
Thus, ![]() is negative if
is negative if![]() , and
, and ![]() if and only if
if and only if ![]() is reduced to the disease-free equilibrium. Consequently, the largest compact invariant set in
is reduced to the disease-free equilibrium. Consequently, the largest compact invariant set in ![]() when
when ![]() is the singleton
is the singleton![]() . Hence, by LaSalle’s invariance principle it implies that “
. Hence, by LaSalle’s invariance principle it implies that “![]() ” is globally asymptotically stable in
” is globally asymptotically stable in ![]() ([14] ).This completes the proof.
([14] ).This completes the proof.
3.6. Existence and Local Asymptotic Stability of Endemic Equilibrium
Since we are dealing with presence of dengue fever disease in human population, we can reduce system (1) to a 3-dimensional system by eliminating![]() ,
, ![]() ,
, ![]() and
and ![]() respectively, in the feasible region
respectively, in the feasible region![]() . The values of
. The values of ![]() can be determined by setting
can be determined by setting ![]() to obtain
to obtain
![]() (10)
(10)
The endemic equilibrium of the system (10) is given by![]() . It is obtained by setting the right hand side of each equation of the system (10) equal to zero which exist for
. It is obtained by setting the right hand side of each equation of the system (10) equal to zero which exist for![]() . Thus, we have
. Thus, we have
![]() (11)
(11)
![]() (12)
(12)
![]() (13)
(13)
3.6.1. Existence of Endemic Equilibrium
For the existence and uniqueness of endemic equilibrium![]() , the conditions
, the conditions ![]() or
or ![]() i.e.
i.e. ![]() or
or ![]() or
or![]() , must be satisfied by using the idea of ([15] ).
, must be satisfied by using the idea of ([15] ).
Adding Equations (11)-(13) above, we have
![]()
or
![]()
But from (13) above
![]()
It follows that
![]()
or
![]()
Consequently
![]()
Then
![]()
This imply that
![]()
and
![]()
meaning that
![]()
Thus, the endemicity of the disease exists since![]() ,
, ![]() ,
, ![]() and
and![]() .
.
3.6.2. Local Stability of the Endemic Equilibrium
In order to analyse the stability of the endemic equilibrium, the additive compound matrices approach is used, using the idea of ([16] ).
If ![]() then the host-vector model Equations (11)-(13) has a unique endemic equilibrium given by
then the host-vector model Equations (11)-(13) has a unique endemic equilibrium given by ![]() in
in![]() , with
, with
![]() (14)
(14)
![]() (15)
(15)
![]() (16)
(16)
Local stability of the endemic equilibrium point is determined by the variational matrix ![]() of the nonlinear system (9) corresponding to
of the nonlinear system (9) corresponding to ![]() as
as
![]() (17)
(17)
The following lemma was stated and proved by [17] , to demonstrate the local stability of endemic equilibrium point![]() .
.
Lemma 1: Let ![]() be the variational matrix corresponding to
be the variational matrix corresponding to![]() . If
. If![]() ,
, ![]() and
and ![]() are all negative, then all eigenvalues of
are all negative, then all eigenvalues of ![]() have negative real parts.
have negative real parts.
Using the above Lemma, we will study the stability of the endemic equilibrium.
Theorem 2: If ![]() the endemic equilibrium
the endemic equilibrium ![]() of the model (10) is locally asymptotically stable in
of the model (10) is locally asymptotically stable in![]() .
.
Proof:
From the Jacobian matrix ![]() in (17), we have
in (17), we have
![]()
Thus, ![]()
Using Mathematica software, we get
![]()
Hence trace and determinant of the Jacobian matrix ![]() are all negative.
are all negative.
The second additive compound matrix is obtained from the following lemma.
Lemma 2: Let ![]() and
and ![]() be subset of
be subset of![]() . The
. The ![]() entry of
entry of ![]() is the coefficient of
is the coefficient of ![]() in the expansion of the determinant of the sub matrix of
in the expansion of the determinant of the sub matrix of ![]() indexed by row in
indexed by row in ![]() and column in
and column in![]() .
.
Proof:
The sub matrix of ![]() is given as
is given as
![]()
The sub matrix of ![]() indexed by rows and columns in
indexed by rows and columns in ![]() is given by
is given by ![]()
The coefficient of ![]() in the determinant of this matrix is
in the determinant of this matrix is ![]() and thus the
and thus the ![]() entry of
entry of![]() is
is![]() .
.
Other entries were done following the same method and to obtain
![]()
Thus
![]()
Using Mathematica software, we get
![]()
Therefore![]() .
.
Thus, from the lemma 1, the endemic equilibrium ![]() of the model system (10) is locally asymptotically stable in
of the model system (10) is locally asymptotically stable in![]() .
.
4. Numerical Simulations
Here, we illustrate the analytical results of the study by carrying out numerical simulations of the model system (1) using the set of estimated parameter values given as shown below.
![]() (18)
(18)
Figure 2 shows that the proportion of Dengue fever disease infectives, treated and recovery proportion of Dengue fever disease all plotted against the proportion of susceptible population. This shows the dynamic behaviour of the endemic equilibrium of the model system (1) using the parameter values in (18) for different initial
![]()
![]()
![]()
Figure 2. Variation of proportion of Dengue fever disease infective population, treated population and recovery population of Dengue fever disease against proportion of susceptibles population.
starting values in three cases as shown below [12] .
![]()
![]()
![]()
The equilibrium point of the endemic equilibrium ![]() was obtained as
was obtained as
![]()
It is observed from Figure 2 that for any starting initial value, the solution curves tend to the equilibrium![]() . Therefore we conclude that the model system (1) is globally stable about this endemic equilibrium point
. Therefore we conclude that the model system (1) is globally stable about this endemic equilibrium point ![]() for the parameters displayed in (18).
for the parameters displayed in (18).
Figures 3(a)-(d) show the variation of population in different classes, human susceptibles, treated human infective, dengue fever patient for different values of ![]() (average daily biting (per day)).
(average daily biting (per day)).
From Figure 3(a), it is observed that proportion of susceptible human population decreases in time slightly before reaching its equilibrium position due to treatment. Therefore, infection becomes less endemic in the pop-
ulation. The proportion of Dengue fever disease infectives decreases in time then reaches equilibrium due to the increase in the number of population getting treatment. Moreover treated infectives increase and then decrease due to infected population moving to other classes, and then also recovery population increases in time as more population are treated. Furthermore infected mosquitoes decrease when the recovery population increases. Mosquito susceptible and aquatics increase with time and reaches its equilibrium point due to its short life span.
From Figure 3(b), it is observed that there is increase in population of dengue fever disease infectives as the value of ![]() increased. If no treatment, large population will die within a very short period of time.
increased. If no treatment, large population will die within a very short period of time.
From Figure 3(c), it is seen that when biting rate increases, the proportional of treated infectives increases and reaches its equilibrium point.
From Figure 3(d), it is observed that when biting rate increase, the proportion of Dengue fever disease increases with time. Therefore protective measures should be taken on board.
Figure 4(a) and Figure 4(b) shows the variation of proportion of dengue fever treated population and recovery population for different values of ![]() (Rate at which dengue fever infected individuals progress for treatment).
(Rate at which dengue fever infected individuals progress for treatment).
From Figure 4(a), it is observed that when treatment is increased, the treated proportion decreases due to treated individuals leaving the class. Moreover when there is no treatment, treated infectives increase and reaches equilibrium due to the decrease of population implying that death rate is high due to lack of treatment.
From Figure 4(b), it is observed that when there is no treatment, i.e.![]() , the dengue fever population decreases. This implies that, death rate increases since there is no treatment. However, when treatment is in progress, a significant increase in the dengue fever recovery (patients) is observed. This also implies that
, the dengue fever population decreases. This implies that, death rate increases since there is no treatment. However, when treatment is in progress, a significant increase in the dengue fever recovery (patients) is observed. This also implies that
![]() (a)
(a)![]() (b)
(b)
Figure 4. Variation of proportion of dengue fever treated population and recovery population for different values of ![]() (Rate at which dengue fever infected individuals progress for treatment).
(Rate at which dengue fever infected individuals progress for treatment).
patients get treatment which prolongs their lives.
5. Discussion and Conclusion
5.1. Discussion
A nonlinear mathematical model has been analysed to study the effect of treatment on the dengue fever disease. The analysis of the model shows that the disease-free equilibrium is locally asymptotically stable by next generation method, which involves the computation of basic reproduction number ![]() less than unity and unstable at reproduction number greater than unity. Then also by using Lyapunov method the disease-free equilibrium point is globally asymptotically stable when
less than unity and unstable at reproduction number greater than unity. Then also by using Lyapunov method the disease-free equilibrium point is globally asymptotically stable when ![]() and unstablewhen
and unstablewhen![]() . Furthermore there exists endemic equilibrium point, that is locally asymptotically stable when
. Furthermore there exists endemic equilibrium point, that is locally asymptotically stable when![]() ,
, ![]() and
and ![]() are all negative, then all eigenvalues of
are all negative, then all eigenvalues of ![]() have negative real parts, where
have negative real parts, where ![]() is the variational matrix corresponding to
is the variational matrix corresponding to![]() .
.
Numerical results are provided to illustrate the analytical results. Sensitivity analysis shows that the average daily biting (per day) ![]() is the most sensitive parameter on
is the most sensitive parameter on ![]() and the least sensitive is the average lifespan of adult mosquitoes (per day)
and the least sensitive is the average lifespan of adult mosquitoes (per day)![]() .
.
In numerical simulation it is observed that the increase of average daily biting (per day), tends to increase the number of infectious individual in the population. But the absence of average daily biting (per day), the infectious population is lowered and the disease can be eradicated. Moreover the increase of treatment will result the reduction of infected proportion as infected proportion population will move to other class, on the other hand when treatment is applied majority of infectious will be observed, as treatment will prolong the life of individual, but with no treatment infectious will be reduced because majority will die and will reach at equilibrium point. From this indicate that there is much work to be done to eradicate the disease by driving reproduction number to be less than unity. Thus the best thing to do is spraying pesticides to kill mosquitoes or sterile male mosquitoes as biological control.
5.2. Conclusion
A compartmental model for Dengue fever disease was presented, a model based on two populations, humans and mosquitoes with treatment. Simulation shows that on the application of treatment, the number of death is reduced. It has been proved algebraically that, if a constant minimum level of a treatment is applied, it is possible to maintain the basic reproduction number below unity, and the infected humans were smaller.
NOTES
*Corresponding author.