Photocatalytic Degradation of p-Nitrophenol (PNP) in Aqueous Suspension of TiO2 ()
1. Introduction
Phenolic compounds discharged in effluents from petrochemicals, agrochemicals, plastic industries, preservative industries, coal distillation plants, pharmaceutical industries etc. are carcinogenic and harmful for the environment. PNP is one of these harmful chemicals and its presence in the environment is a threat for living beings. Many investigations have been carried out regarding the removal of PNP from the aqueous media [1] -[3] . In homogeneous system H2O2, O3 or Fentons reagents and UV radiation are used for the oxidative degradation of organics. The process of using H2O2 under UV radiation has the advantages having low capital cost, no solid waste and no undesirable vapor emission through or after the oxidation. In heterogeneous system, semiconductor mediated-photodegradation of PNP has been investigated [4] . A number of photocatalysts such as TiO2, ZnO, Fe2O3, ZnS, WO3 and CdS are used for this purpose.
Chen and Ray studied the photo catalytic degradation of phenol, PNP and 4-nitrophenol (4-NP) in aqueous suspension and over immobilized Degussa P25 TiO2 in the laboratory [5] . They conducted the experiments to investigate the effect of catalyst dose, pollutant concentration, temperature, partial pressure of oxygen, intensity of UV light, catalyst-layer thickness, circulation flow rate and catalyst annealing temperature. The investigators observed pseudo first-order kinetics with respect to all the parent compounds. San et al. studied the kinetics of the photocatalytic degradation of PNP in the presence of TiO2 [6] . They examined the effects of H2O2 and Cu(II) ions on degradation rate and found that the addition of H2O2 increased the reaction rate while Cu(II) ions suppressed the degradation rate. TSA Islam and her co-workers investigated the effect of pH, ions and ionic strength on TiO2-mediated photodegradation of Brilliant Orange and found that addition of Pb(II) ion in suspension enhanced the degradation [7] . The aim of the present investigation is to find out the experimental conditions under which PNP molecules will be completely mineralized.
2. Experimental
Commercial PNP was used for experiments without further purification. Titania P-25 (anatase) 99.0% purity was obtained from Fluka. Electrolytes used in the experiments were obtained from Merck.
Experiments were carried out using 100 mL of 0.5 × 10−4 M PNP solution in suspension of TiO2, the concentration of which was varied from 2 g/L to 8 g/L. pH was recorded before irradiation. The suspension was stirred magnetically and irradiated. The irradiated samples were collected after definite intervals of time and were analyzed. The experimental procedure is given elsewhere [7] . The absorption spectra of irradiated samples were recorded by UV-visible spectrophotometer (UV-1610A, Shimadzu, Japan) at λmax = 313 nm.
The percent degradation (%) has been calculated as follows:
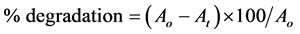
where Ao is the initial absorbance and At is the absorbance of the sample irradiated for t minutes.
Photodegradation experiments were repeated to investigate the effect of concentrations of TiO2 (Figure 1 and Figure 2) and PNP in the suspension (Figure 3 and Figure 4). After photodegradation, the pH of suspension was always found to be lower than the initial pH. In separate experiments the pH was monitored during mineralization by dipping the pH-electrode in suspension and the photodegradation was carried out until the pH reached steady value.
3. Results and Discussion
3.1. Effect of Concentration of TiO2 Suspension on Photodegradation of PNP
Figure 1 shows how the degradation of PNP changes with increasing concentrations of TiO2 suspension. The concentration which corresponds to the maximum degradation of PNP was 0.4 g/100 mL. As the concentration of TiO2 in the suspension was increased, the adsorption site on the surface was increased. As a result the extent of adsorption of PNP on the TiO2 surface was increased and the percent degradation was also increased. With the continuous increase of concentration of TiO2 in the suspension, the adsorption increased until the surface was likely to be saturated but percent degradation did not increase. The reason is UV light would face difficulties to penetrate and reach the surface under these conditions. As a result, percent degradation decreased. The present observations are in agreement with earlier observations [8] -[10] .
3.2. Effect of the Initial Concentration of PNP on Photodegradation
The photodegradation was investigated by varying the initial concentration of PNP over the range of 0.5 × 10−4 M to 2.5 × 10−4 M. Figure 2 shows five different initial concentrations of PNP solutions and values of absor-

Figure 1. Effect of concentration of TiO2 in the suspension on photodegradation of PNP at concentration of 1.0 × 10−4 M and pH 3.0.
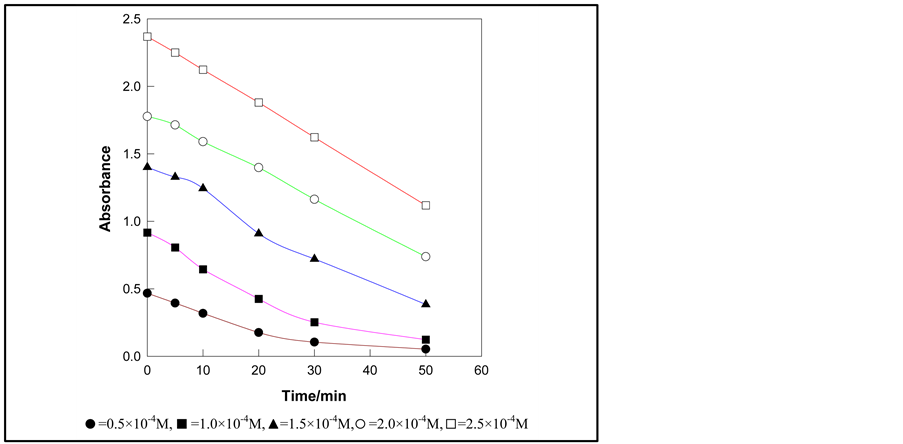
Figure 2. Absorbance change of PNP with times of irradiation at different initial concentrations of PNP in suspension[s] containing 0.4 g TiO2/100mL and solution pH 3.0.
bance at different intervals of time. Figure 3 shows the change of percent degradation of PNP solution with increasing PNP concentration at definite interval of time. It is evident that percent degradation of PNP decreases with increasing concentration. This can be explained by the possible photocatalytic reactions taking place on the surface of TiO2. An approximately single layer of adsorbed water molecule exists on the surface of TiO2. Of the two types of carriers formed after light is adsorbed on the surface of TiO2, h+ oxidizes water molecules producing .OH radicals which have strong oxidizing power. These radicals can react with PNP producing free radicals. These free radicals can react with molecular oxygen producing organic peroxy radicals which can propagate a chain reaction. PNP molecules are completely degraded within a short time.
 (1)
(1)
 (2)
(2)
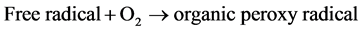 (3)
(3)
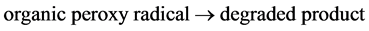 (4)
(4)

Figure 3. Effect of initial concentration of PNP on photodegradation. [TiO2] = 0.4 g/100 mL. Solution pH = 5.0.
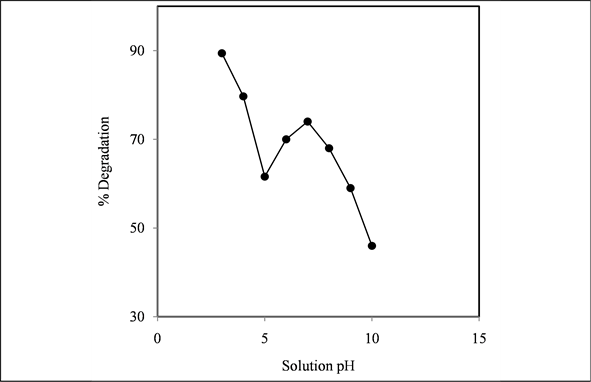
Figure 4. Effect of solution pH on photodegradation of PNP. [TiO2] = 0.4 g/100 mL, [PNP] = 1.0 × 10−4 M.
With increasing of PNP concentration more PNP molecules accumulate on the surface by replacing water molecules [11] . As a result, reaction 1 slows down resulting in low production of  radicals. Neppolian et al. [12] found similar observations with some commercial textile dyes under solar irradiation. The present observations are also in good agreement with the results obtained by a group of researchers, who have investigated photocatalytic degradation of dyes in aqueous solution using TiO2 suspension [13] -[20] .
radicals. Neppolian et al. [12] found similar observations with some commercial textile dyes under solar irradiation. The present observations are also in good agreement with the results obtained by a group of researchers, who have investigated photocatalytic degradation of dyes in aqueous solution using TiO2 suspension [13] -[20] .
3.3. Effect of pH of the Suspension on Photodegradation of PNP
Solution pH is an important factor that regulates aqueous-phase semiconductor mediated photocatalytic reaction. It affects dissociation of substrate, surface charge, conduction band potential of semiconductor [7] and other physicochemical properties of the system.
Figure 4 shows the percent degradation of PNP with increasing pH of the suspension. The maximum photodegradation was observed in highly acidic solution. As the pH increases from 3 to 5, degradation decreases. But surprisingly, the rapid increase of degradation was observed as pH was increased from 5 to 7. Beyond 7.5, the degradation was found to decrease. The pHZPC of TiO2 is 6.25 and in acidic condition, the surface pH is lower than pHZPC of TiO2 [11] . As a result in acidic condition surface of TiO2 becomes positively charged. PNP is strongly adsorbed on TiO2 surface due to the presence of lone pair of electron in phenolic OH group. This might be the reason for maximum degradation in acidic solution. As the pH increases, surface loses its positive character and degradation decreases. When the pH exceeds 7.5 meaning that the solution is alkaline, the surface becomes negative and PNP remains as anion under these conditions. Obviously PNP molecules have difficulties to approach the surface to be adsorbed. This might be the reason for decreasing photodegradation in alkaline solution. However, neutral region favoured the photodegradation. Under these conditions, adsorption of either PNP or PNP- would decrease. It is expected that the rate of production of .OH radical by the reaction  would increase. Increase of production of .OH radical would increase the photooxidation. Islam et al. and her co-workers investigated the effect of pH, ions and ionic strength on TiO2-me- diated photodegradation of Brilliant Orange which is an anionic dye, and they found that the degradation rate was high in acidic media and low in the region of pH 6 to 7 [7] .
would increase. Increase of production of .OH radical would increase the photooxidation. Islam et al. and her co-workers investigated the effect of pH, ions and ionic strength on TiO2-me- diated photodegradation of Brilliant Orange which is an anionic dye, and they found that the degradation rate was high in acidic media and low in the region of pH 6 to 7 [7] .
3.4. Effect of Cation on the Photodegradation of PNP
The effect of cations on the photodegradation was studied by monitoring the hydrogen ion concentration of the solution during irradiation. At the beginning, two experiments were carried out, one using only the TiO2 suspension (blank) and the other one using PNP in the suspension. The irradiation was carried out by adding different concentrations of Cu(II) and Fe(II) ions in the suspension of PNP and in the blank, followed by monitoring of H+ ion concentrations at different time intervals. Figure 5 shows the change of H+ ion concentrations with time under different experimental conditions.
Any metal ion will be adsorbed on the TiO2 surface if the reduction potential of the metal ion is higher than the conduction band potential of TiO2. The conduction band potential of TiO2 is −0.45 V for pH 5.95 and the redox potentials of Fe(II) (aq)/Fe(s) and Cu(II) (aq)/Cu(I) (aq) are −0.41 and 0.16 V respectively.
The ions adsorbed on the TiO2 particle can trap an electron to form Ti-O-M.
This might be oxidized by oxygen at the interface producing superoxide anion.

The highly reactive superoxide radical anions attach itself to the peroxyl radicals formed from the organic compounds as mentioned earlier in reaction 3 producing an unstable product which contains at least four oxygen and this can decompose into carbon dioxide molecule. Thus the superoxide anions play an important role in enhancing the rate of mineralization. However, addition of excess ions decreased H+ ion concentration. Excess ions occupy the adsorption sites on the surface of TiO2 and thus decreased the adsorption of PNP on the TiO2

Figure 5. Effect of cations on the photodegradation of PNP. 1 = no PNP (blank), 2 = PNP only, 3 = PNP with 1 × 10−9 M Fe(II), 4 = PNP with 1 × 10−5 M Cu(II), 5 = PNP with 1 × 10−5 M Fe(II), 6 = PNP with 1 × 10−3 M Fe(II), 7 = PNP with 1 × 10−3 M Cu(II).
surface. As a result, photodegradation and mineralization decreased. This observation is in good agreement with the previous findings [7] [21] .
Hua et al. [21] studied the effect of presence of Cu(II) ions on the photodegradation of monocrotophos and found that at low concentration of Cu(II) (<10−5 mol∙dm−3) slight enhancement of photodegradation was observed whereas at higher concentration photodegradation was decreased. Zang et al. [22] investigated the effect of Cu(II) ion on 2,4-DCP and showed that the photodegradation of 2,4-DCP was increased for the addition of Cu(II) ion at low concentrations but in presence of higher concentrations of Cu(II) it decreased.
3.5. Effect of Anions on Photodegradation of PNP
To investigate the effect of anions on photodegradation of PNP, the experiments were carried out using sodium salts of ,
,  ,
,  and
and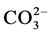 . All these anions decreased the percent degradation of PNP. These observations are in good agreement with the previous results [7] .
. All these anions decreased the percent degradation of PNP. These observations are in good agreement with the previous results [7] .
4. Conclusion
TiO2 mediated photodegradation at 254 nm light for 2 hours showed that about 90% of PNP was degraded in 0.4 g/100 mL of TiO2 suspension containing 1.0 × 10−4 M of PNP. Addition of Cu(II) and Fe(II) ions in TiO2-suspension enhances the degradation of PNP suggesting that the presence of metal ions has higher redox potential than the conduction band potential of TiO2 and accelerates the mineralization, but the presence of excess ions decreases the mineralization process. The complete mineralization of 1.0 × 10−4 M PNP may be obtained by using 0.4 g/100 mL of TiO2 suspension with either 10−9 M Fe(II) or 10−5 M Cu(II) as impurities and illuminating for 2 to 3 hours or until steady increase of H+ ion concentration is observed.
Acknowledgements
Authors would like to acknowledge to the Ministry of Science and Technology of the People’s Republic of Bangladesh for the partial financial support to carry out this work.
NOTES
*Corresponding authors.