The genus of Silene (Caryophyllaceae) is widely distributed in Uzbekistan and it is known as the rich source of phytoecdysteroids. The one main of them is the ecdysterone, which is possessed as an anabolic effect for human body [2]. The ecdysterone has the ability to adapt organism to experimental effects of environment and stimulate the operability [3].
The “Ecdisten” preparation is produced from the medicinal plants Raponticum and Silene at the Institute of the Chemistry of Plant Substances (Tashkent, Uzbekistan Academy of Sciences).
2. RESULTS
In the present paper the chemical composition of Silene praemixta plant was investigated. We described the isolation and structure elucidation of ecdysteroids from the waste product of this manufacture.
Four ecdysteroids were isolated and identified from Silene praemixta (1-3) and 22-O-β-D-glucopyranoside of 2-deoxyecdysone (4) (Figure 1).
Part of the waste extract (6.0 g) was subjected to column chromatography on silica gel with gradient elution by CH3Cl-MeOH (10:1), CH3Cl-MeOH-H2O (70:12:1) and (70:23:4) to afford 1-4. Steroids 1-2 were the main compounds of the plant extract while 3-4 compounds were found in scarce amount.
The identification of isolated compounds was carried out by using 1D and 2D NMR spectroscopy techniques: 1H, 13C, DEPT, 1H-1H COSY, HSQC, NOESY, IR spectroscopy and ESI MS. This spectral data identified steroid 1 as 2-deoxyecdysone, steroid 2 as 2-deoxyecdysterone, steroid 3-ecdysterone [4-7] and compound 4 as 22-O-β-D-glucopyranoside of 2-deoxyecdysone [8].
3. DICUSSION
Using infrared spectrophotometer data we obtained information regarding presence of hydroxyl groups (3387 - 3409 sm−1) and 6-ketochromaphore (1639 - 1652 sm−1) of tested compounds according to ecdysteroids skeleton. The mail role in the structure determination of isolated compounds plays NMR analysis which shows presence of signals of methyl groups at 0.64 and 1.57 ppm, corresponding to CH3-18, CH3-19 (CH3-21 represented as doublets in area 0.88 - 1.6 ppm). Signals of CH3-18 determined by presence of Δ7-6-keto-14α-oxygroup, and signals at C-7 in current system shown in area 5.75 - 6.25 ppm. All mentioned data distribute identification of isolated compounds 1-4 as 2-deoxy-α-ecdysone, 2-deoxyecdysterone, ecdysteron (3), 22-О-β-D-glucopyranosid- 2-deoxy-α-ecdysone. All chemical shifts of mentioned compounds correspond to literature data [4-8].
4. EXPERIMENTAL
General Methods
1H, 13C NMR (Table 1) spectra were run in CD3OD, C5D5N using TMS as internal reference on a Bruker Avance DRX 600 MHz spectrometer. ESI-MS spectra were recorded on a PE Q-STAR electrospray, ionization time of flight-tandem-mass spectrometry spectrometer. Silica gel 60 PF 254 was used for TLC. Infrared spectra were taken on Perkin Elmer Spectrum 100 FTIR sprectrophotometer, which are conformed with literature data.
2-Deoxy-α-ecdysone (1)-C27H44O5
1Н NMR 2-deoxy-α-ecdysone (400 МHz, C5D5N, δ, ppm, J/Hz, 0-TMS): 0.73 (CH3-18, s), 1.05 (CH3-19, s), 1.30 (CH3-21, d, 3J = 6.6), 1.39 (CH3-26 and CH3-27, s), 2.97 (H-17, m), 3.50 (H-9, m), 4.07 (H-22, m), 4.15 (H-3, w.s), 5.95 (H-7, d, 4).
1Н NMR 2-deoxy-α-ecdysone (400 МHz, CD3OD, δ, ppm, J/Hz, 0-HМDS): 0.66 (CH3-18, s), 0.88 (CH3-21, d, 3J = 6.8), 0.90 (CH3-19, s), 1.13 (CH3-26, s), 1.14 (CH3-27, s), 2.36 (H-5, dd, 12, 4), 3.14 (H-9, m), 3.54 (H-22, w.d), 3.92 (H-3, m), 5.75 (H-7, d, 2.3).
IR spectrum 2-deoxy-α-ecdysone (KBr, νmax, sm−1): 3387, 1640.
ES-MS Positive ion mode of 2-desoxy-α-ecdysone: 471.2 [M + Na]+.
ES-MS negative ion mode of 2-desoxy-α-ecdysone 447.1 [M − H]−.
2-Deoxyecdysterone (2)-C27H44O6
1Н NMR 2-deoxyecdysterone (600 МHz, C5D5N, δ, ppm, J/Hz, 0-TMS): 1.06 (CH3-19, s), 1.24 (CH3-18, s), 1.37 (CH3-26 and CH3-27, s), 1.60 (CH3-21, s), 3.04 (H-17, t, 9), 3.54 (H-9, m), 3.89 (H-22, w.d, 10.2), 4.12 (H-3, w.s), 6.25 (H-7, s).
1Н NMR 2-deoxyecdysterone (400 МHz, CD3OD, δ, ppm, J/Hz, 0-HМDS): 0.82 (CH3-18, s), 0.90 (CH3-19, s), 1.13 (CH3-21 and CH3-26, s), 1.14 (CH3-27, s), 2.05 (H-12, td, 12.9, 5), 2.35 (H-5 and H-17, m), 3.15 (H-9, m), 3.27 (H-22, m), 3.93 (H-3, m), 5.73 (H-7, d, 2.4).
IR-spectroscopy of 2-deoxyecdysterone (KBr, νmax, sm−1): 3388, 1639.
ES-MS Positive ion mode of 2-deoxyecdysterone 487.3 [M + Na]+.
ES-MS negative ion mode of 2-deoxyecdysterone 463.0 [M − H]−.
Ecdysteron (3)-C27H44O7
1Н NMR ecdysteron (600 МHz, C5D5N, δ, ppm J/Hz, 0-TMS): 1.06 (CH3-19, с), 1.22 (CH3-18, s), 1.36 (CH3-26 and CH3-27, s, 1.59 (CH3-21, s), 3.00 (H-5 and H-17, m), 3.59 (H-9, m), 3.87 (H-22,w.d, 10.2), 4.18 (H-2, m), 4.22 (H-3, w.s), 6.25 (H-7, d, 1.8).
1Н NMR ecdysteron (400 МHz, CD3OD, δ, ppm, J/Гц, 0-HМDS): 0.83 (CH3-18, s), 0.90 (CH3-19, s), 1.126 (CH3-21, s), 1.134 (CH3-26, s), 1.38 (CH3-27, s), 2.32
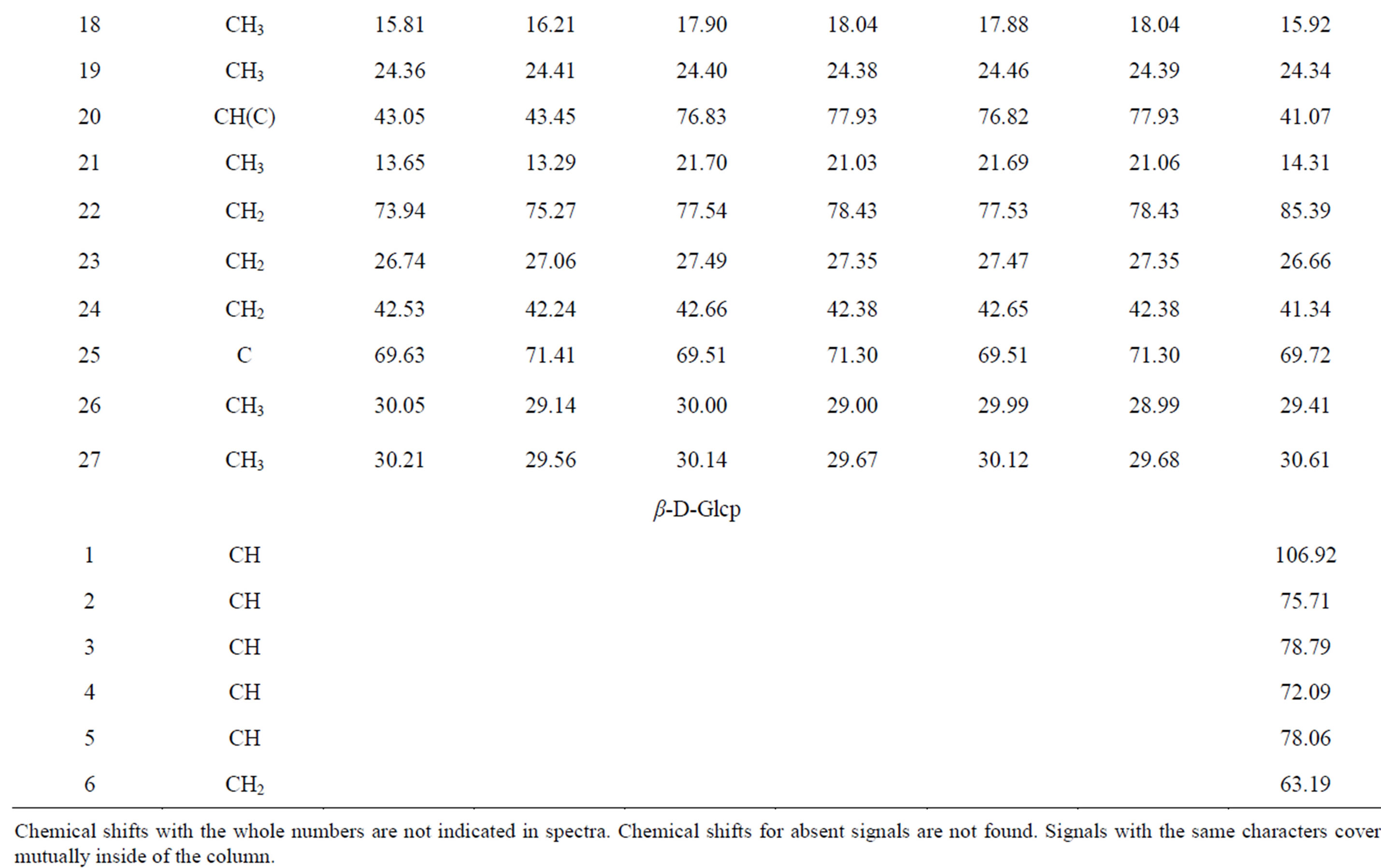

Table 1. 13C NMR spectra compounds of 1-4 (C5D5N, CD3OD, δ, ppm, 0-TMS).
(H-5 and H-17, m), 3.09 (H-9, m), 3.30 (H-22, m), 3.76 (H-2, m), 3.88 (H-3, m), 5.75 (H-7, d, 2.5).
IR-spectrum ecdysteron (KBr, νmax, sm−1): 3409, 1652.
ES-MS Positive ion mode of ecdysteron: 503.3 [M + Na]+.
ES-MS negative ion mode of ecdysteron: 479.1 [M − H]−.
22-О-β-D-Glucopyranosid-2-deoxy-α-ecdysone (4)- C33H54O10
Spectroscopy 1Н NMR 22-О-β-D-Glucopyranosid-2- deoxy-α-ecdysone (400 МHz, C5D5N, δ, ppm, J/Hz, 0-HМDS): 0.64 (CH3-18, s), 0.91 (CH3-19, s), 1.08 (CH3-21, d, 3J = 6.7), 1.19 (CH3-26, s), 1.23 (CH3-27, s), 3.36 (H-9, m), 3.86 (H-5 Glu, m), 3.98 (H-3, H-22 and H-2-Glu, m), 4.15 (H-3 Glu and H-4 Glu, m), 4.30 (H-6 Glu, dd, 2J = 11.4, 3J = 5.4), 4.47 (H-6’ Glu, dd, 2J = 11.4, 3J = 2.7), 4.92 (H-1 Glu, d, 3J = 7.7), 6.07 (H-7, d, 3J = 2).
5. CONCLUSION
Compound 22-О-β-D-Glucopyranosid-2-deoxy-α-ecdysone (4) was isolated from Silene praemixta M. Pop. for the first time.
ACKNOWLEDGEMENTS
This study was supported by the National Key Projects for Science and Technology Development of AS Uzbekistan during the five year plan period (FA-F3-T044)