A Green Protocol for Efficient Synthesis of 1,8-Dioxo-Octahydroxanthenes Using Ionic Liquid ()
1. Introduction
1,8-dioxo-octahydroxanthenes are important class of oxygen heterocycles in which a phenyl substituted pyran ring is fused on either side with two cyclohexanone rings. Presence of conjugated bis-dienone functionality makes these compounds sensitive to be attacked by nucleophiles and light energy. In the past decade, synthesis of xanthenes derivatives has been of considerable interest to organic chemists because they possess various biological and pharmaceuticals activities such as antiviral [1], antibacterial [2] and anti-inflammatory [3] properties. These are being utilized as antagonists for paralyzing action of zoxazolamine [4] and in photodynamic therapy [5]. Xanthenes and benzoxanthenes derivatives are the parent frame works found in a large number of naturally occurring as well as synthetic products possessing prominent position in medicinal chemistry [6,7]. Xanthenediones are likewise special structural units constituting various natural products [8] and being used as versatile synthons, because of inherent reactivity of their inbuilt pyran ring [9].
Apart from their use as valuable synthetic precursors for many organic compounds [10] and dyes [11], they are also used in laser technologies [12] and pH—sensitive fluorescent materials for the visualization of biomolecules assemblies [13].
There are a wide variety of methods for the preparation of the xanthenes having been reported and they are classified according to starting compounds which includes synthesis by cyclization of polycyclic aryltiflate esters [14], intra-molecular trapping of benzynes by phenols [15] and reaction of aryloxy magnesium halides with triethyl orthoformate [16] as well as cyclo-condensation of 2-hydroxyl aromatic aldehyde with 2-tetralone [17].
One of the commonly used methods reported for the synthesis of xanthenediones involves the condensation of aromatic aldehydes with 1, 3-cyclohexanedione or 5,5- dimethyl-1, 3-cyclohexanedione. The various catalysts have been reported for synthesis of 1,8-dioxo-octahydroxanthens: NSPVPC [18], [Et3NH][HSO4] [19], SiCl4 [20], [Et3NC4SO3H] [HSO4]/Al2O3 [21], polyaniline p-toluenesulfonate [22], alumina-sulfuric acid [23], CAN [24], [BMIm][BF4]-Mg(BF4) [25], I2 [26], [Et3N-SO3H]Cl [27], DABCO [28], SmCl3 [29], cellulose sulfonic acid [30], heteropolyacid-MCM-41 i.e. (H3PW12O40) [31], [bmim] HSO4 [32], Fe(HSO4)3 [33], [Hbim]BF4 [34], TBAHS [35] tetramethylguanidium trifluoroacetate [36], [DDPA] [HSO4] [37], [TMPSA] HSO4 [38], [Hmim] TFA [39], trichloro isocyanuric acid [40], SbCl3/SiO2 [41], SiO2-RSO3H [42], InCl3/P2O5 [43], p-dodecyl benzenesulfonic acid [44], triethyl benzyl ammonium chloride (TEBA) [45], diammonium hydrogen phosphate [46], PPA-SiO2 [47], montmorillonite K-10 [48], Fe3+-Montmorillonite [49], trimethyl silyl chloride [50], NaHSO4-SiO2 [51], ZnO-Acetyl chloride [52], PMA-SiO2 [53], ZrOCl2∙8H2O [54], Amberlyst-15 [55], Dowex-50W [56]. Most of these methods have some advantages and disadvantages, taking the weakness into consideration such as long reaction time, low yields, use of hazardous catalysts and solvents, tedious work up processes and difficulty in recovery and reusability of catalyst. To overcome these problems we have developed the green protocol for efficient synthesis of 1,8-dioxo-octahydroxanthenes using [bmim]ClO4 ionic liquid (Scheme 1).
In recent years, ionic liquids have emerged as a useful alternative to conventional organic solvents and catalysts due to their particular properties, such as negligible vapor pressure, chemical stability, excellent solvent power for organic and inorganic compounds, as well as the ease of recovery [57]. Recently, much attention has been paid to organic reactions carried by using ionic liquids greater than ever importance in the context of green synthesis. Although ionic liquids were primarily introduced as an alternative green reaction medium, today they have progressed extremely beyond that, playing a significant role in controlling reactions as catalysts.
2. Experimental Procedure
All the chemicals were purchased from Sigma Aldrich and Merck chemicals. Melting points recorded on Veego melting point apparatus and were uncorrected. 1H NMR spectra were recorded on a Brucker Avance II 400 MHz spectrometer with TMS as internal standard. IR spectra were determined on Perkin Elmer Spectrum RX FTIR spectrometer. The ionic liquid was synthesized according to ref. [58].
General Procedure for Synthesis of 1,8-Dioxo-Octahydroxanthens Derivatives
To a mixture of aldehyde (1 mmol) and 5,5-dimethyl- 1,3-cyclohexanedione (2 mmol) and [bmim]ClO4 (4 mmol) was added in 50 ml round bottom flask and was stirred at 100˚C on heating magnetic stirrer (Scheme 1). The progress of the reaction was monitored by TLC after completion of the reaction the reaction mixture was cooled to room temperature and water (5 ml) was added, solid separated was filtered. The crude product was recrystallized from ethanol to give the pure product. The [bmim]ClO4 was recovered by distillation and reused 3 times.
The comparison of various reported catalyst is summarized in (Table 1).
3,3,6,6-tetramethyl-9-(4-chloro-phenyl)-1,8-dioxo-
octahydroxanthene (Entry 1) IR (cm−1): 3030, 2963, 2952, 1679, 1661, 1469, 1361, 1198, 1166, 1003, 852. 1H NMR: 7.17 - 7.24 (dd, 4H), 4.71 (s, 1H), 2.46 (s, 4H), 2.18 (q, 4H), 1.10 (s, 6H), 0.98 (s, 6H). 13C NMR: 191.10, 157.17, 137.45, 126.79, 124.53, 122.97, 110.03, 45.45, 35.61, 26.96, 26.22, 24.03, 22.05 m/z: 385.2 (M + 1), 273.2.
3,3,6,6-tetramethyl-9-(4-methyl-phenyl)-1,8-dioxo-
octahydroxanthene (Entry 2) IR (cm−1): 3036, 2959, 2873, 1663, 1623, 1359, 1197, 1164, 1139, 1000, 842. 1H NMR: 7.17 (d, 2H), 7.01(d, 2H), 4.71(s, 1H), 2.45 (s, 4H), 2.24 (s, 3H), 2.18 (q, 4H), 1.09 (s, 6H), 0.99 (s, 6H). 13C NMR: 196.4, 162.0, 141.1, 135.7, 128.7, 128.2, 115.7, 50.7, 40.8, 32.2, 31.4, 29.2, 27.3, 21.0. m/z: 365.3 (M + 1), 273.2.
9,9’-(1,4-phenylene)bis(3,3,6,6-tetramethyl-3,4,5,6,7, 9-hexahydro-1H-xanthene-1,8 (2H)-dione (Entry 4) IR (cm−1): 2957, 2871, 1665, 1620, 1365, 1201, 1167, 1144, 1005, 590. 1H NMR: 7.07 (s, 4H), 4.70 (s, 2H), 2.42 (s, 8H), 2.17 (s, 8H), 1.07 (s, 12H), 0.97 (s, 12H). m/z: 13C NMR: 196.4, 162.4, 141.7, 127.9,115.7, 50.8, 40.8, 32.2, 30.1, 28.9, 27.7. m/z: 645.4 (M + Na), 273.2.
3,3,6,6-tetramethyl-9-(4-hydroxy-3-methoxy-phenyl)- 1,8-dioxo-octahydroxanthene (Entry 5) IR (cm−1): 3412, 3029, 2954, 1666, 1622, 1514, 1431, 1359, 1278, 1229, 1197, 1135, 1028, 624, 571. 1H NMR: 7.01 (s, 1H), 6.73 (d, 1H), 6.56 (dd, 1H), 5.49 (bs, 1H, -OH), 4.66 (s, 1H), 3.89 (s, 3H), 2.45 (s, 4H), 2.20 (q, 4H), 1.00 (s, 12H). 13C NMR: 196.6, 162.0, 145.8, 144.0, 136.4, 120.0, 115.8, 113.9, 112.2, 55.8, 50.7, 40.8, 32.2, 31.3, 29.3, 27.2. m/z: 396.47, 419.3 (M + Na), 273.2.
3,3,6,6-tetramethyl-9-(4-methoxy-phenyl)-1,8-dioxooctahydroxanthene (Entry 7) IR (cm−1): 3059, 2958, 2876, 1665, 1626, 1511, 1462, 1357, 1260, 1193, 1109, 1031, 841, 569. 1H NMR: 7.18 (d, 2H), 6.75 (d, 2H), 4.69 (s, 1H), 3.73 (s, 3H), 2.45 (s, 4H),1.09 (s, 6H), 0.99 (s, 6H). 13C NMR: 196.5, 162.0, 157.9, 136.4, 129.3, 115.7, 113.4, 55.1, 50.7, 40.8, 32.2, 30.9, 29.2, 27.3. m/z: 381.2, 273.2.
3. Result and Discussion
To a mixture of 4-chloro benzaldehyde (1 mmol) and 5,5-dimethyl-1,3-cyclohexanedione (2 mmol) and [bmim] ClO4 (4 mmol) was added in 50 ml round bottom flask and was stirred at 100˚C on heating magnetic stirrer. The progress of the reaction was monitored by TLC After completion of the reaction the reaction mixture was cooled to room temperature and water (5 ml) was added, solid separated was filtered and product was characterized by IR, NMR, 13C-NMR and mass.
The reaction between dimedone and 4-chloro benzaldehyde in the presence of [bmim]ClO4 was studied as a model reaction. To optimize the reaction conditions of reaction temperature we have tried the study of above model reaction from temperature 60 - 110˚C in the gap of 10˚C. The best result in terms of time and yield for the reaction has been observed at 100˚C (Table 2).

Scheme 1 . Synthesis of 1,8-dioxo-octahydroxanthene using [bmim]ClO4 ionic liquid.

Table 1. Comparison table with various reported catalysts.
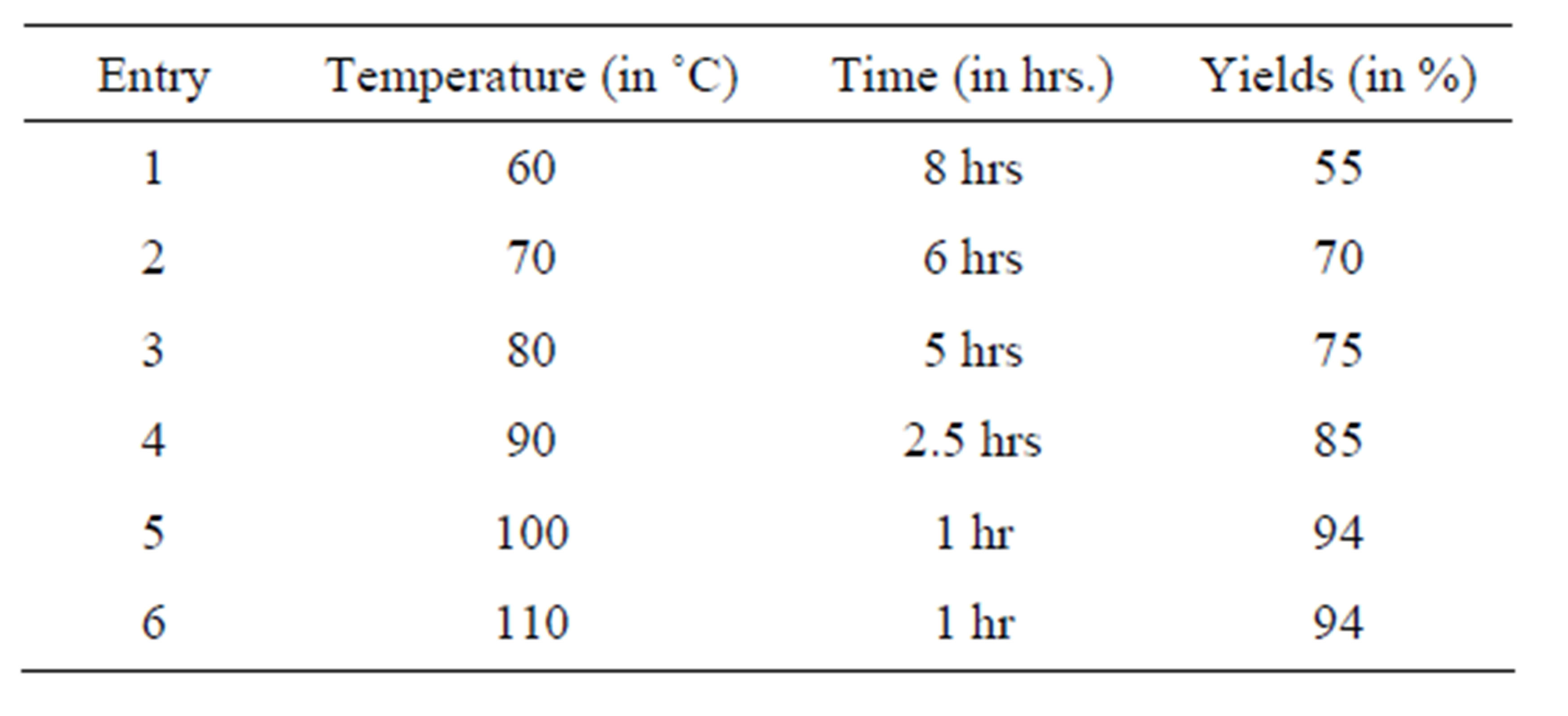
Table 2. Optimization of reaction temperature for synthesis of 1,8-dioxo-octahydroxanthenes.
To evaluate the scope of this reaction a range of 1, 8-dioxo-octahydroxanthenes were prepared by the reaction of dimedone and aromatic aldehydes under optimized reaction conditions. The results are summarized in the table (Table 3). Various aromatic aldehydes with electron donating substituent’s reacted efficiently and quickly with dimedone to give cyclocondensation products in high yields over short reaction times. Similarly aldehydes with electron withdrawing substituent’s reacted efficiently to yield the corresponding 1,8-dioxooctahydroxanthenes.
Study of reuse of the ionic liquid can be easily done up to 3 times with excellent yields without loss in the reactivity (as shown in graph/Figure 1). After 3rd cycle we observed sudden decrease in the 4th reuse of ionic liquid thus the ionic liquid used was recycled for 3 times. The structure of isolated products was assigned based on their

Table 3. Green protocol for efficient synthesis of 1, 8-dioxoxanthenes using [bmim]ClO4.a
spectral analyses as well as by matching with their melting point with reported analogous.
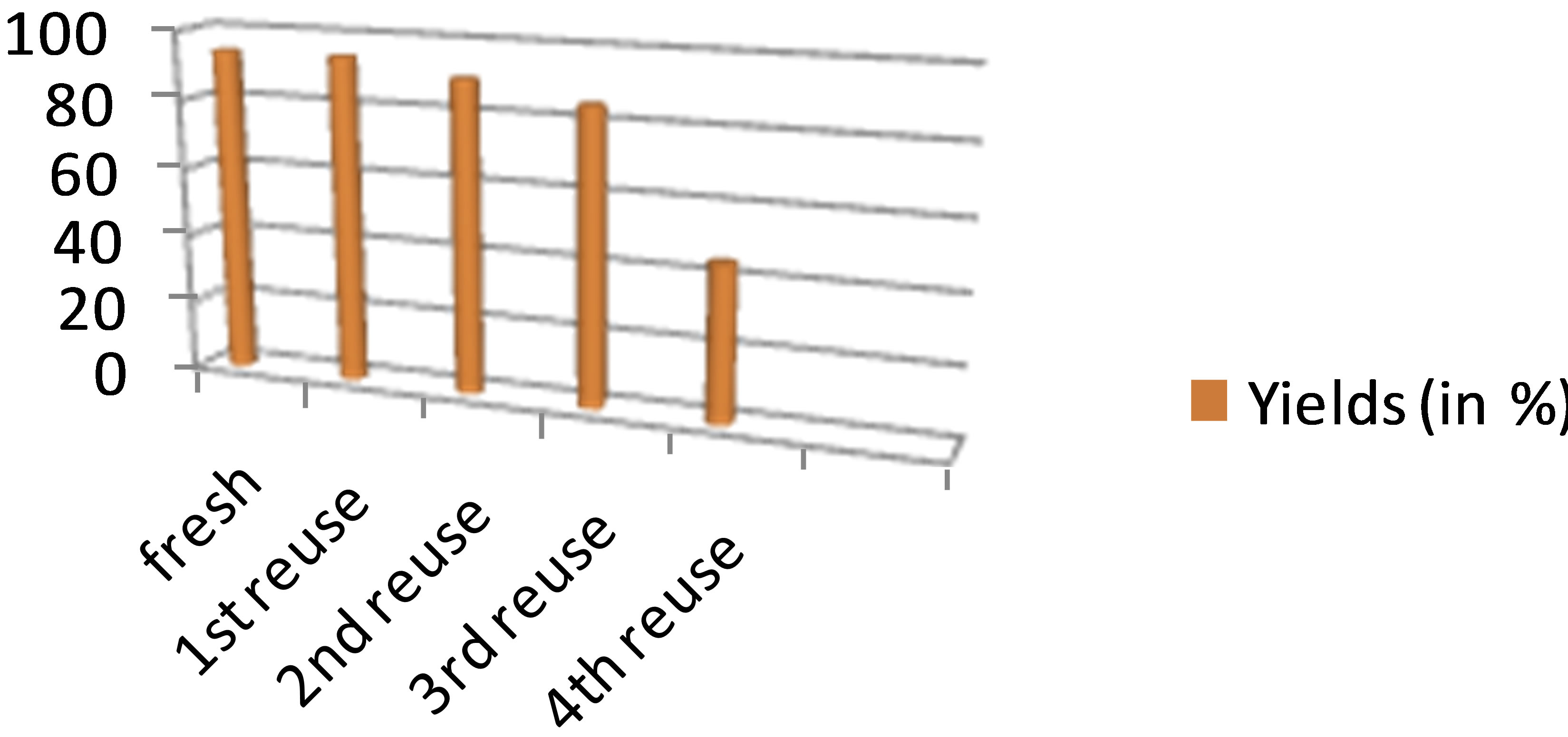
Figure 1. Study of reuse of ionic liquid.
4. Conclusion
In summary, we have developed a high-yielding simple, convenient, straight-forward and practical one-pot procedure for the synthesis of different types of 1,8-dioxooctahydroxanthenes derivatives using [bmim]ClO4. The ionic liquid used can be easily recovered and reused 3 times without significant decrease in the yield of the product.
NOTES