Pathogenic Bacteria Associated with Different Public Environmental Sites in Mecca City ()
1. Introduction
Scientific research has shown that commonly used surfaces such as computers, telephones, headsets, desks and ATM machines are potential sources of infectious bacteria and viruses leading to the spread of colds, flu, sickness and diarrhea [1-3]. They are constantly in contact with the environment wherever we go. Germs can survive in the microscopic grooves and cracks on surfaces and will go unnoticed. Oils in the skin, dust, grime, moisture and warmth from central heating systems provide an ideal environment for these germs to accumulate.
Cold and flu viruses can survive on dry surfaces for more than 48 hours, while some bacteria, such as E. coli, can survive on dry surfaces for months on end. Soft, wet surfaces (particularly those with plenty of food) are perfect for bacteria. Cloth, sponges and carpets that have gotten wet are excellent living places for bacteria because it protects them from exposure to the environment, dry air or sunlight [4].
Bacteria that can cause severe gastroenteritis have been found on ATM machine keypads. Roxburgh [5] demonstrate that germs can be readily transferred from your hands to almost any frequently used surface. The role of fomites in the transmission of disease remains a controversial subject. Some epidemiological studies have suggested that contaminated surfaces may play a role in the spread of respiratory viruses and laboratory studies have supported this hypothesis [6-8]. Other studies have implicated environmental surfaces in the transmission of bacteria [2,9,10]. However, the role of environmental surfaces in the transmission of disease remains an issue of scientific debate and fundamental information concerning the microbial transfer rates from environmental surfaces to the hands and from the hands to the mouth remains scarce. Aim of this manuscript was to evaluate the presence or absence of pathogenic bacteria from different public sites in Mecca, SA.
2. Materials & Methods
2.1. Samples Collection
Samples were collected from the following sites in Mecca city: 1) Swabs of shopping trolleys in supermarkets; 2) Swabs of the existing holes in the glass fish market suburb; 3) Swabs from the handles of the doors of ATM; 4) Swabs of refrigerated water taps in the streets; 5) Swabs from the mike, pens and keyboards in the public halls; 6) Swabs from handles laundry in public restaurants; and 7) Swabs from the surfaces of cans of carbonated drinks.
2.2. Transportation of the Samples
All samples were collected on Amies transport swabs media and transported to the microbiology research laboratory without any delay.
2.3. Cultivation of Samples
All samples were collected processed in the research laboratory according to the standard microbiological methods under complete aseptic conditions. The swabs were inoculated on MacConkey agar (Oxoid, England), blood agar, bile esculin agar (Oxoid, England), mannitol salt agar (Oxoid, England) andnutrient agar (Oxoid, England) and incubated at 37˚C under aerobic conditions.
2.4. Isolation and Identification of Bacteria
All bacteria were isolated and identified according to the conventional microbiological methods as described in Burnett and Crocker [11], and Goldman and Green [12].
2.4.1. Staphylococcus aureus
Staphylococcus aureus was isolated from mannitol salt agar after an overnight incubation as a mannitol fermenting colonies. The identification was confirmed microscopically by the characteristic appearance as Gram positive cocci in clusters after being stained by Gram stain. Catalase test was also performed to differentiate between Staphylococcus (catalase positive) and Streptococcus (catalase negative). Then coagulase test was also performed to differentiate between Staphylococcus aure us (coagulase positive) and Staphylococcus spp. (coagulase negative).
2.4.2. Pseudomonas aeruginosa
Pseudomonas aeruginosa was isolated from nutrient agar medium which was grown after overnight incubation. The identification was confirmed by the characteristic appearance as colonies with greenish coloration and microscopically as Gram negative bacilli after being stained by Gram stain. An oxidase test was also performed, in which the organism is characteristic positive.
2.4.3. Enterococcus faecalis
Enterococcus faecalis isolated from Bile esculine agar medium which was grown after overnight incubation period. The identification was confirmed by the characteristic appearance as black colonies and microscopically Gram positive cocci in chains after being stained by Gram stain. Lancefield grouping test was also performed as group D.
2.4.4. Bacillus spp.
Bacillus spp. was isolated from Blood agar medium which was grown after an overnight incubation period. The identification was confirmed microscopically as Gram positive bacilli in chains.
2.4.5. Biotyping of Enterobacteriaceae Isolates by API 20E System
The API 20E system (Bio-Mérieux, Marcy l’Etoile, France) isan identification system for Enterobacteriaceae and other non-fastidious gram-negative rods. This system consists of 20 microtubes containing dehydrated substrates. These tests are inoculated with a bacterial suspension which reconstitutes the media. During incubation, metabolism produces color changes that are either spontaneous or revealed by the addition of reagents. The reactions are read according to the table for interpretation of reactions and the identification is obtained by referring to the Analytical Profile Index (API).
Procedure:
1) Preparation of the inoculums A single well-isolated colony was selected from an isolation plate and carefully emulsified in sterile NaCl 0.85% medium, pH 5.5 - 7.0 to achieve a homogeneous bacterial suspension.
2) Inoculation of the strip With a pipette, the tests of Citrate utilization, Acetoin production and Gelatinase were filled with the bacterial suspension. Anaerobiosis was created in the tests Arginine dihydrolase, Lysine decarboxylase, Ornithine decarboxylase, H2S production and Urease by overlaying with mineral oil. The incubation box of the strip was closed and incubated at 35˚C - 37˚C for 18 - 24 hours.
3) Reading the strip After the incubation period 18 - 24 hours at 35˚C - 37˚C, the strip was read by referring to interpretation of reactions. All spontaneous reactions were recorded on the result sheet.
3. Results
The quantitative analysis of all tested samples was 648, of which 422 was negative bacterial count (71%). Alternatively, total positive samples were 226 (29%) of counted bacteria Table 1. However, 100% positive results were recorded of samples collected from the glass windows in the fish market and 14% negative results of swabs from the surfaces of cans of carbonated drinks. This is indicate that refrigeration keep out bacteria away. The most obvious way of solving the problem bacterial contamination is to keep places cool even glass windows in the fish market; also, is to regularly clean and disinfect the glass.
Contaminated sites were more frequently in some sites such as shopping cart handles, inner surfaces and child seats in supermarkets (n = 48), and refrigerated water taps in the streets (n = 47) than other sites; surfaces of cans of carbonated drinks (n = 13) and from handles laundry in public restaurants (n = 26). The most negative contaminated site was mike, pens and keyboards in the
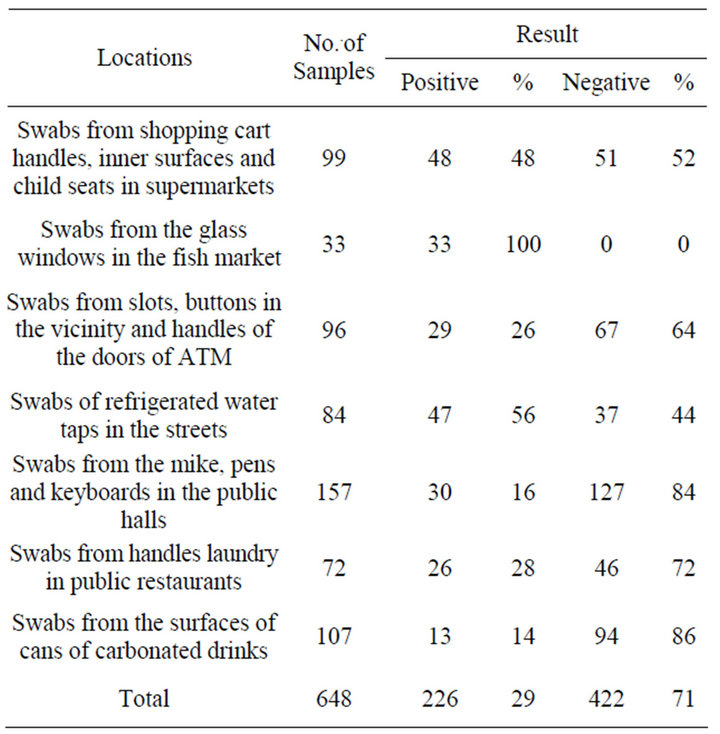
Table 1. Frequency and percentage of positive and negative collected samples.
public halls (n = 127) (Figure 1). Unexpectedly, more than half of samples collected from shopping trolleys in supermarkets were negative (n = 51) for presence of bacteria at all. A particular result could regard to just cleaning a trolleys in time or other reason not founded interpretation.
Quantitative bacterial analysis of isolates indicated presence of 226 bacterial isolates obtained from shopping cart handles, inner surfaces and child seats in supermarkets (n = 48); from the glass windows in the fish market (n = 33); from slots, buttons in the vicinity and handles of the doors of ATM (n = 29); of refrigerated water taps in the streets (n = 47); from the mike, pens and keyboards in the public halls (n = 30); from handles laundry in public restaurants (n = 26); from the surfaces of cans of carbonated drinks (n = 13). Table 2 shows per-

Figure 1. Positive and negative contaminated samples.
centage of each infected site by Gram-positive or Gramnegative bacteria in relation to species type. From first inspection percentage of Bacillus spp. recorded the highest percentage between other isolated bacteria. This might due to their nature as spore former which could tolerate adverse conditions as well as flourish in normal circumstances.
All isolated bacteria were tested on blood agar medium to detect the potential pathogenic bacteria. Isolated Gram-negative bacteria (8 species) were varied from hemolytic bacteria (Acinetobacter haemolyticus), histamine producer (Morganella morganii), multidrug resistance (Pseudomonas aeruginosa) to coliform group (E. coli, Enterobacter aerogenes and Citrobacter freundii). These bacteria found in public sites can pose serious health risks if uncontrolled. E. coli recorded 8 isolates of 48 positive contaminated sites (Figure 2).
There are several obvious differences between sites which bacteria were isolated from Mecca. The population density, the structures present and the atmospheric variability are all starkly diverse along this gradient, but we looked to address our hypothesis that the current cleaning regiment at the facilities and flat surfaces are not sufficient.
Bacillus spp. recorded 97 isolates from all sites where the highest one was from the mike, pens and keyboards in the public halls (n = 19) followed by the handles of the doors of ATM, from refrigerated water taps in the streets and from handles laundry in public restaurants were 18, 17 and 16 isolates, respectively. Enterococcus faecalis isolates detected in high rate at refrigerated water taps in the streets and shopping trolleys in supermarkets where 15 and 14 were, respectively (Figure 3).
4. Discussion
This study concentrated mainly on pathogenic bacteria for the aim of work and not includes plate bacterial count for the tremendous number of collected samples. Many

Figure 2. Speciation of gram-negative bacteria in relation to infected sites.

Table 2. Percentage of bacterial diversity isolated from different sites in Mecca.
investigators have been studied solid surface bacterial infection in computer keyboard [13], mobile phones [14], computer keyboards and mice, elevator buttons and shopping carts [15] and currency notes [16]. They were not collected such huge samples number as in our case (648 samples).
A low positive percentage (16%) results were detected from samples collected from the mike, pens and keyboards in the public halls. In contrast, Sheet of Protech IT hygiene (Technical release/162) [17] reported that there can be up to 400 times more bacteria on office desks and keyboards than toilet seats. In our view of point, the place of which samples collected, hygienic status of keyboards, cool of hall atmosphere could make such a difference.
Datta et al. [18] have isolated only Gram-positive bacteria Staphylococcus, Enterococcus, Micrococcus and

Figure 3. Speciation of Gram-positive bacteria in relation to infected sites.
Streptococcus from mobile phones. In this study a variety of media used in isolation process to fulfill the requirement of bacteria from different sites. It is obvious that large number of isolated Bacillus species (18 of 29 positive samples from slots, buttons in the vicinity and handles of the doors of ATM and 19 of 30 positive samples from the mike, pens and keyboards in the public halls) was transferred from finger tips or hands touching inanimate surfaces.
In comparison to many other studies, Yazah et al. [16] and Catãno et al. [19] obtained Gram-positive and Gramnegative bacteria in their work from currency notes and computer keyboards, curtains, cell phones, white coats, and ties, respectively. Such regarding results were typical to our results. Likewise, regular cleanness of contaminated sites with different disinfectants can minimize bacterial growth; it is extremely difficult to completely eliminate all bacteria from surfaces.
5. Acknowledgements
The authors would like to thank third-year students in the faculty of Medicine, umm Al-Qura University, Mecca, Saudi Arabia, for their cooperation in sample collection. Thanks also to Mr. Abdullah Apyeed and Mr. Assem Abdel-Shakoor for their assistance during this study.