Studies on Extraction Behaviour of Cobalt(II) with Nitrobenzoylprazolone-5 ()
1. Introduction
Available reports on solvent extraction methods for cobalt (Co) appear to focus more on the use of organophosphorus compounds or extractants [1-4] and little has been published on the use of pyrazolones; and that is why this study is important. Pyrazolones are prominent analytical reagents [5-8] and potent drugs or pharmaceutical agents [9-12]. The importance of these compounds lies in their ability to form complexes with many metal ions under varied experimental conditions. Of the aroyl pyrazolones, 1-phenyl-3-methyl-4-benzoylpyrazol-5-one (HPMBP) and its substituted analogues have been used as extractants for isolation and separation of various metals [13-15] especially the Group II and transition metals. In our search for various uses of 5-pyrazolones, we have found HPMNP most promising, simple and selective reagent for Co(II). Extraction studies of metals using this reagent have not appeared widely in the literature. The versatility of HPMNP is comparable to the more popular chelating extratants, HPMBP and Thenoyltrifluoroacetone (TTA), but surpasses them in terms of relative ease of preparation, storage stability and ability to precipitate and extract most metal ions at relatively wide range of pH values [8,16-17]. The purpose of this study is to investigate the dependency of extraction of Co(II) on the usual extraction parameters such as pH, extractant concentration and nature of the organic solvents using this reagent, and to determine the possible stoichiometry of the extracted species.
2. Materials and Methods
2.1. Materials
2.1.1. Preparation of HPMNP
The process involved benzoylation of 1-phenyl-3-methyl-pyrazolone-5 (HPMP) with p-nitrobenzoyl chloride, by dissolving HPMP (17.6 g, 0.100 mole) in dioxane (75 ml) with gentle warming in a 500 ml three-necked roundbuttom quick-fit flask equipped with a magnetic stirrer, separatory funnel, and a reflux condenser. Calcium hydroxide (7.5 g, 0.103 mole) was added dropwise within 2 - 5 minutes (which had been previously dissolved in 50 ml of dioxane). The mixture was continuously stirred, and gently refluxed for 90 minutes till the yellow calcium complex decomposed by pouring into 400 ml of chilled 3 M HCl, whereby cream nitro-benzoylpyrazolone-5 separates.
Re-crystallization of the crude product was from an ethanol-water mixture containing a little HCl to destroy any un-decomposed calcium complex. The crystals are orange-yellow; yield, 86%; and m.pt, 160.5˚C. This method has been adopted by Uzoukwu [18], Meckler [13] for the synthesis of other related acylpyrazolones. The reagent exhibits a keto-enol tautomerism and can be obtained in both forms. The orange-yellow enol form was obtained by re-crystallization from heptane or warm ethanol-water mixture, while the colorless keto form was obtained from hot water. No differences in behaviour of these two forms were observed. In the present work, the enol form was used. The HPMP was prepared as recorded by Okafor [19]. Microanalyses for N, H, and C were performed to confirm the purity of the reagent. All other reagents were of analytical grades, and were used without further purification.
2.1.2. Preparation of Stock Solutions
Solutions of HPMNP were made by dissolving an appropriate amount of it in organic solvent, so that the stock solution was 0.02 M. A Stock solution of 1.7 × 10−3 M Co(II) was prepared by dissolving 0.48 g of CoSO4·7H2O in 100 ml of 0.1 M H2SO4. Further dilutions were made from it as required.
2.2. Methods
2.2.1. Extraction Procedures
To 5 ml of an aqueous buffer solution containing 2 ppm of Co(II) was added 5 ml of 0.02 M solution of HPMNP in chloroform. The two phases were mechanically shaken on a Stuart flask shaker for 60 minutes at 26˚C ± 0.5˚C. Preliminary experiments show that 30 minutes were sufficient for equilibration. For pH studies, the solutions of Co(II) were adjusted to different pH values using chlorides (KCl/HCl), actate (NaAc/HAc) and borate buffer solutions to cover pH 1 to 8. The equilibrated phases were allowed 30 minutes time, and the concentration of Co(II) remaining in the aqueous phase was determined spectrophotometrically using 1-nitroso-2-naphthol. Hydrogen peroxide was used as the oxidizing agent, followed by extraction with toluene and measuring at 410 nm. The aqueous phase in each case was maintained at a constant ionic strength of 0.3 M with sodium sulphate, Na2SO4·10H2O. The pH values were measured with a digital pH meter Model HM-208 while a Pye Unicam SP8-100 spectrophotometer was used for spectrophotometric measurements.
2.2.2. Variation of Extractant Concentration
5 ml aliquots of HPMNP of varying concentrations in chloroform were equilibrated with equal volumes (5 ml) of aqueous phase of fixed pH 5.5 (with NaAc/HAc buffer) and ionic strength of 0.3 M. The solutions were allowed to settle for half an hour in order to separate the phases after which suitable aliquots of the aqueous phase were taken for analysis of Co(II).
3. Results and Discussion
HPMNP is a beta-diketone and exists in both the keto and the enol forms. Figure 1 shows the enol (a) and keto (b) forms of this reagent. The HPMNP enolate ion forms highly extractable metal chelate of the form shown in Figure 1(c), where n is the charge on the uncomplexed metal ion, and is equal to the number of ligands coordinated to the metal. For the case of Co(II) being considered, n = 2. The presence of heterocyclic rings containing
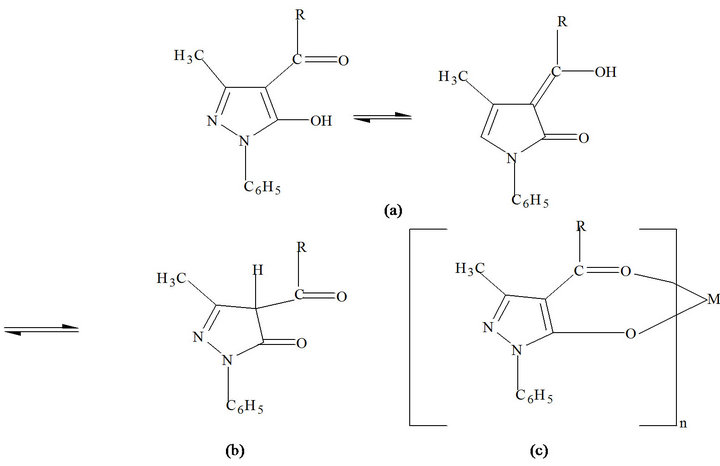
Figure 1. Keto-enol tautomerism (R = nitrophenol).
N atoms and the nitro groups in the pyrazolone ligands seem to increase the stability of the resulting metal chelates; otherwise, their properties are expected to be paralleled to those of other metal acylpyrazolonates.
3.1. Effect of pH
Figure 2 shows the extraction plots obtained by plotting the log of distribution ratio, D against pH when HPMNP is dissolved in chloroform. At lower pH values, the plots are linear with slopes of 1.98 and 2.02 for extractant concentrations of 0.02 M and 0.03 M respectively. The distribution ratio (D) of Co(II) increased with pH up to a maximum value of 5.5. Between the pH 5.5 and 7.0, Co(II) is constantly and quantitatively extracted into the organic phase. The extraction of Co(II) regressed at higher pH values where metal hydrolysis is believed to play a dominant role. The slope of approximately 2.0 for the plots indicated that D is inverse second-power dependent on hydrogen ion concentration of the aqueous phase at room temperature, and that two moles of H+ ions were released per mole of Co(II) ion on formation of an extractable complex (See Equation (3)).