Investigation of Surface Free Energy for PTFE Polymer by Bipolar Argon Plasma Treatment ()
1. Introduction
Polytetrafluoroethylene (PTFE) is a polymer used as insulator in cables, connector assemblies and for printed circuit boards because of its good dielectric properties [1]. Nowadays in medical products and surgical instruments, polymeric devices are improving current healthcare practice. The polymeric devices are meeting the requirements of biocompatibility between the physiological environment and the biomaterial surface. Mostly PTFE is used as vascular grafts in cardiovascular applications. In many applications where good adhesion between a polymer and a coating is necessary, it must be modified to assure better adhesiveness. The best method for modifying the surface properties of materials is the plasma treatment. [2-6]. The plasma treatment can affect only the first few nanometers of material without changing the bulk properties [7]. It can cause chemical bonding of oxygen atoms at active sites on the polymer surface, leading to formation of various functional groups that modify the surface wettability.
The plasma treatment usually does not produce only one type of a functional group on a polymer surface. Hence, it is necessary to apply such plasma that facilitates formation of the functional groups that are most important for a given application. The increase in treatment times used for surface modification of polymers causes oxidation, removal of surface contaminants and improving of surface wettability. Long treatment time can cause chemical etching, increase in sample temperature, creation of nanostructures and irreversible damage of the bulk properties. The main drawback of plasma treatment for activation of polymers is ageing. The various functional groups formed during plasma treatment are not stable with time. Hence, the surface tends to approach to its untreated state. Hence, the surface keeps loosing its hydrophilic character spontaneously [8]. The fastest method to check the effect of plasma treatment on surface is wettability by measuring the contact angles of a suitable liquid drops with a well known surface energy (water and glycerin).
In this article, we present a study on surface modification of PTFE film by a bipolar argon plasma treatment. The surface properties of PTFE such as surface free energy, morphology, chemical composition, micro hardness and adhesion were investigated.
2. Experimental
Experiments were performed with PTFE films of thickness 450 μm, which was cut to a small pieces of size 2.0 × 2.0 cm2. PTFE samples were cleaned in isopropyl alcohol and dried before inserting into the plasma chamber. The set up consists of 60 cm long cylindrical chamber of 30 cm diameter. It has two rectangular parallel plates of stainless steel of dimensions 16 cm × 7.5 cm, which works as electrodes. The inter electrode gap was maintained 2 cm in all the experiments. The Schematic of the experimental setup is as shown in Figure 1.
Bipolar pulsed power source was used to generate the desired argon plasma [9]. The applied Voltage and current were measured with the help of high voltage probe (Tektronix P6015A, 1000X) and current transformer respectively. Tektronix (TDS 2024, 200 MHz) digital oscilloscope was used to record the voltage and current waveforms. The chamber pressure was 0.1 mbar during the argon plasma treatment. The samples were treated in argon plasma for 5, 10 and 50 minutes respectively.
The surface morphology was studied by atomic force microscope (Digital Nanoscope IIIa Instrument Inc.) in contact mode. Digital Microhardness Tester (FM 700, Future-Tech Corporation, Kawasaki-Japan) was used to determine the Vicker’s hardness number (Hv) by the indentation technique. Contact angle goniometer (NRL C.A. Goniometer, Model 100-00-230) was used to measure the contact angle. XPS measurements on the above samples were carried out using VSW ESCA machine with monochromatic AlKα radiation (1486.6 eV) [10]. During the experiment, chamber vacuum was better than 10–9 torr. The electron take-off angle was 40˚ and the X-rays were operated at 10 KV and 10 mA emission current. To compensate for the charging effects, the carbon peak assumed to be lying at 284.8 eV. Curve fitting has been done using the Gaussian/Lorentzian curve fitting programme [10].
3. Results and Discussion
3.1. Determination of Surface Energy
A good understanding of the surface properties of a solid may be obtained relatively inexpensively from the measurement of the surface free energy. Therefore, the contact angle measurement has been used in the study of surface free energy, wettability and adhesion of low surface energy materials [11,12]. The surface free energy of a solid
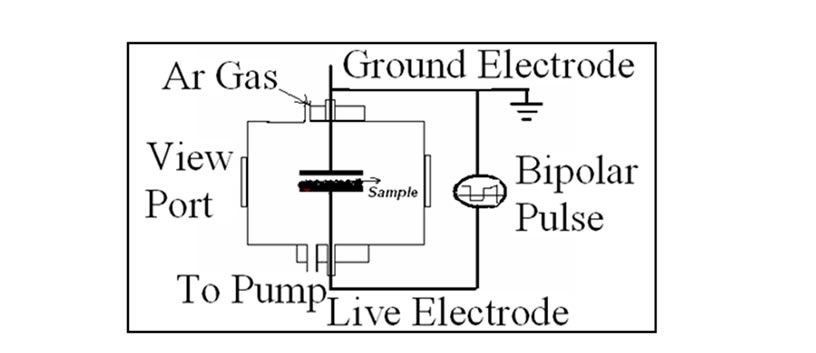
Figure 1. Schematic of the experimental set-up.
is an important parameter, playing a vital role in the phenomena that occur at solid-liquid and solid-gas interfaces. Hence, knowledge of this parameter is useful in the studies of adsorption and wettability processes, which play important role in many industrial applications of the material. Measurement of contact angle of liquid with the solid surface permits a rapid and qualitative evaluation of surface free energy of polymer. In the present paper, analysis of the surface free energy of PTFEs has been made on the basis of dispersive and non-dispersive components. Surface free energy (γs) and its polar ( ) and dispersion (
) and dispersion ( ) components of the sample were determined from two sets of contact angles (water and glycerin) according to Owens-Wendt-Kaelble equation (Owens and Wendt 1969) [13].
) components of the sample were determined from two sets of contact angles (water and glycerin) according to Owens-Wendt-Kaelble equation (Owens and Wendt 1969) [13].
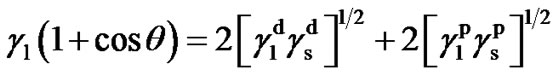
where,  ,
,  and
and  are the total surface free energy, the polar component and the dispersion component of the surface free energy of the liquid, respectively. The values of the surface free energies of the test liquids obtained from the literature are given in Table 1 [14].
are the total surface free energy, the polar component and the dispersion component of the surface free energy of the liquid, respectively. The values of the surface free energies of the test liquids obtained from the literature are given in Table 1 [14].
The water contact angle of untreated sample was about 76˚. With an increasing treatment time, it was decreased to about 60˚. This shows the improvement in wettability of PTFE surfaces. Similarly, the contact angle for glycerin was measured on PTFE surfaces and it changes from 81˚ to 60˚ with increasing treatment time. The values of surface free energy and its components before and after the treatment are compared in Figure 2. Since PTFE is a chemically inert polymer, plasma treatment has little
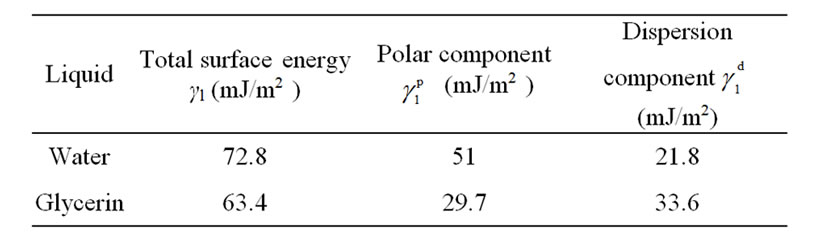
Table 1. Surface free energy and its polar and dispersion components of water and glycerine used to determine the surface free energy of PTFE.
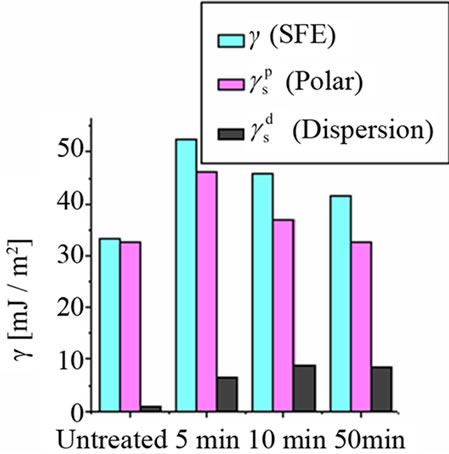
Figure 2. Comparison of surface free energy and its components before and after the treatment in Ar discharge.
effect on surface activation i.e increase in surface energy. The increase in surface free energy is attributed to the functionalization of the polymer surface with hydrophilic groups on the surface.
For untreated PTFE, the value of polar component is comparable to SFE and dispersion component is not appreciable. There is a little increase in the polar component after all subsequent treatments, whereas remarkable change occurred in the dispersion component. This slight change in polar component is due to the chemically inert nature of PTFE. An important information obtained from the surface energy measurement is that the increase in polar component indicates the formation of covalent bonds.
3.2. Microhardness
The Vicker’s hardness number (Hv) was determined by the indentation technique performed with a microhardness tester in the load range 10 to 500 gf for a constant loading time of 10 s. Indentation was made with a Vickers’ diamond pyramidal indenter housing a square base and pyramidal angle of 136˚ between the opposite faces attached to an optical microscope using a pilar micrometer/ image analyzer. The average value of diagonal of indentation was used for the calculation of hardness value. The hardness number (Hv) was calculated using the relation:
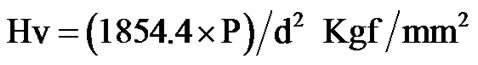
where P is the indenter load in gf, d is the average of the two diagonal lengths in micrometers. Several indentations were obtained at each load and the average hardness number was calculated. The variation of Hv with load for both untreated and argon plasma treated PTFEs is illustrated in Figure 3.
The Vicker’s hardness increases as load increases. However, on approaching a certain load value, the rate of increase of hardness slow down and then became constant. At higher loads, beyond 200 gf, the interior of the bulk specimen is devoid of surface effects. Hence hardness value at higher loads represents the true value of the bulk and is consequently independent of the load. The
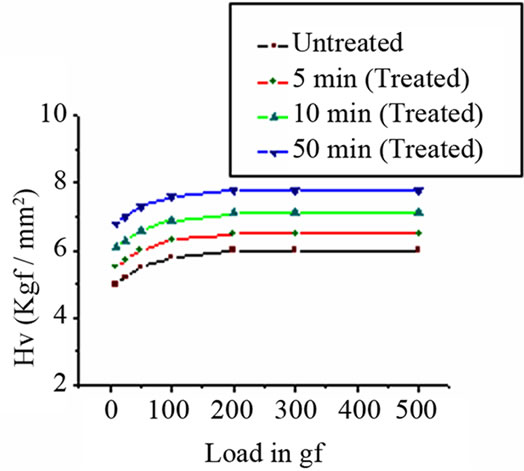
Figure 3. Plot of hardness vs applied loads.
hardness is found to increase as treatment time increases. This may be attributed to cross-linking phenomenon on the polymer surface [15-17]. It is also corroborated with XPS analysis.
3.3. XPS Analysis
A suitable method for the determination of type of functional groups on a polymer surface is X-ray photoelectron spectroscopy (XPS). For detailed analysis of chemical changes induced by plasma treatment on PTFE surfaces, high resolution XPS spectra were recorded as shown in Figures 4(a)-(f).
A basic problem in polymer analysis is surface charging due to a loss of surface electrons by X-ray irradiation. In common practice manual shifting of unfunctionalised C 1s peak (C-C) to 284.8 eV is performed. From the recorded spectra, it can be noticed that there is little change in shape of the curve of untreated and treated samples. The C 1s peak for treated and untreated samples does not show much variation in area under the peak. Similarly, comparison of fluorine peak does not show significant difference. The surface composition of PTFE before and after the plasma treatment is tabulated in Table 2.
The F/C ratio decreases with increase in treatment time, but the O/C ratio increases due to defluorination and oxi-
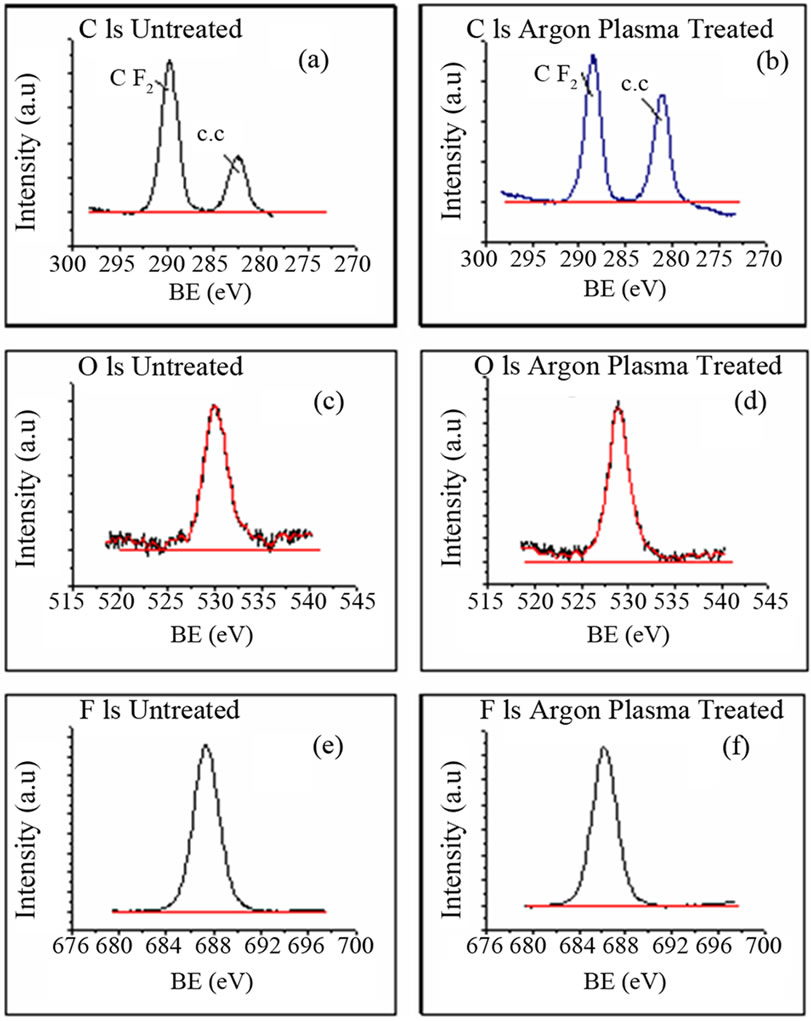
Figure 4. XPS spectra of (a) C 1s untreated; (b) C 1s argon plasma treated; (c) O 1s untreated; (d) O 1s argon plasma treated; (e) F 1s Untreated; (f) F 1s argon plasma treated.
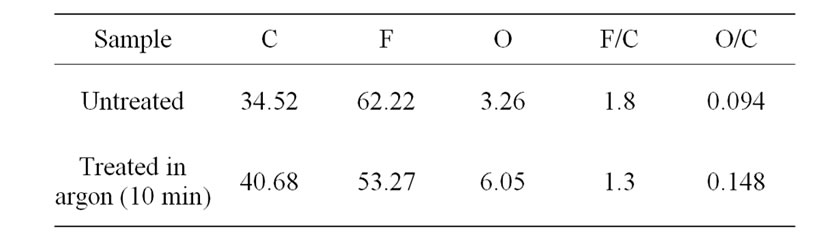
Table 2. The surface composition of PTFE before and after the plasma treatment.
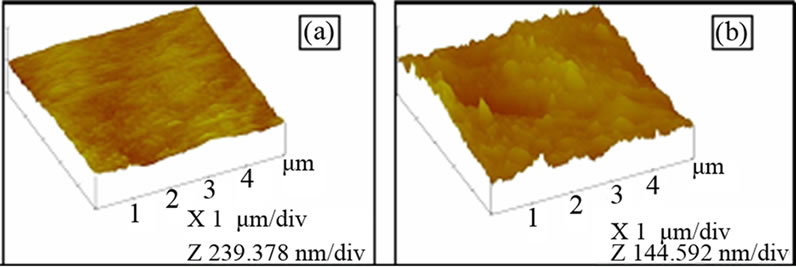
Figure 5. AFM Photomicrograph of (a) Untreated PTFE film; (b) Argon plasma treated PTFE for 10 min.
dation on surface. This indicates the formation hydrophilic group such as C-O on the surface [10].
3.4. Atomic Force Microscopy (AFM)
The surface morphology of argon treated PTFE samples were measured by AFM in contact mode on a 5 × 5 µm2 area and are shown in Figures 5(a)-(b). Each AFM image was analyzed in terms of surface average roughness (Ra). The data show that the average surface roughness (rms) increases with treatment time. The average surface roughness (rms) for untreated film and argon plasma treated film for 10 min are 8.5 nm and 22.8 nm respectively. The roughness of the PTFE surfaces increases with treatment time, hence it can support the adhesion improvement [18]. This result is corroborated with surface free energy results.
4. Conclusion
The increase in surface free energy indicates the improvement of adhesion on polymer surface. This is supported by AFM results as roughness increases. The XPS analysis reveals an increase in O/C ratio due to plasma treatment i.e. surface contains hydrophilic functional group. The hardness increases with increase in treatment time.
NOTES